In the poultry industry, antibiotic growth promoters (AGPs) have been used for decades to enhance gut health and control sub-clinical diseases (Abreu et al., 2023 ![]() ; Abreu et al., 2023
; Abreu et al., 2023 ![]() ). However, the use of synthetic growth enhancers and supplements presents several challenges. These additives are expensive, often unavailable, and can have adverse effects on both birds and humans (Abd El-Hack et al., 2022
). However, the use of synthetic growth enhancers and supplements presents several challenges. These additives are expensive, often unavailable, and can have adverse effects on both birds and humans (Abd El-Hack et al., 2022 ![]() ). Sub-therapeutic levels of antibiotics given to poultry as growth enhancers can result in the development of antibiotic-resistant bacteria, posing significant hazards to animal and human health (Kasimanickam et al., 2021
). Sub-therapeutic levels of antibiotics given to poultry as growth enhancers can result in the development of antibiotic-resistant bacteria, posing significant hazards to animal and human health (Kasimanickam et al., 2021 ![]() ).
).
Concerns about AGPs impact on human health have led to global restrictions (Salim et al., 2018 ![]() ). AGPs work by interacting with the intestinal microbial population, improving nutrient absorption, reducing toxin production, and decreasing subclinical infections (Broom, 2017
). AGPs work by interacting with the intestinal microbial population, improving nutrient absorption, reducing toxin production, and decreasing subclinical infections (Broom, 2017 ![]() ; Krajmalnik‐Brown et al., 2012
; Krajmalnik‐Brown et al., 2012 ![]() ).
).
The use of antibiotics as feed additives has come under severe criticism due to the risk of promoting antibiotic-resistant bacteria, which threatens human health (Ben et al., 2019 ![]() ; Ma et al., 2021
; Ma et al., 2021 ![]() ; Manyi-Loh et al., 2018
; Manyi-Loh et al., 2018 ![]() ). Concerns have been raised that the use of antibiotics for therapeutic and growth promotion purposes could lead to increased resistance in bacteria of both human and animal origin, particularly gram-negative bacteria such as Salmonella spp. and Escherichia coli (Butaye et al., 2003
). Concerns have been raised that the use of antibiotics for therapeutic and growth promotion purposes could lead to increased resistance in bacteria of both human and animal origin, particularly gram-negative bacteria such as Salmonella spp. and Escherichia coli (Butaye et al., 2003 ![]() ; Rahman et al., 2022
; Rahman et al., 2022 ![]() ). Consequently, the poultry industry is moving towards reducing the use of synthetic antibiotics (Mehdi et al., 2018
). Consequently, the poultry industry is moving towards reducing the use of synthetic antibiotics (Mehdi et al., 2018 ![]() ; Selaledi et al., 2020
; Selaledi et al., 2020 ![]() ).
).
In response to these concerns, the European Union (EU) has banned the use of antibiotic growth promoters as additives in animal nutrition (Castanon, 2007 ![]() ). This has led to the exploration of alternative feed additives, referred to as Natural Growth Promoters (NGPs) or non-antibiotic growth promoters. These include acidifiers, probiotics, prebiotics, phytobiotics, feed enzymes, immune stimulants, and antioxidants (Ayalew et al., 2022
). This has led to the exploration of alternative feed additives, referred to as Natural Growth Promoters (NGPs) or non-antibiotic growth promoters. These include acidifiers, probiotics, prebiotics, phytobiotics, feed enzymes, immune stimulants, and antioxidants (Ayalew et al., 2022 ![]() ; Kikusato, 2021
; Kikusato, 2021 ![]() ). Plant materials, rich in bioactive compounds, have been used for medical treatment since prehistoric times and are now gaining attention for their potential to positively affect poultry health and productivity (Ivanova et al., 2024
). Plant materials, rich in bioactive compounds, have been used for medical treatment since prehistoric times and are now gaining attention for their potential to positively affect poultry health and productivity (Ivanova et al., 2024 ![]() ; Jamil et al., 2022
; Jamil et al., 2022 ![]() ).
).
Plants contain important bioactive components such as alkaloids, flavonoids, glycosides, saponins, phenols, terpenoids, essential oils, and polypeptides. These compounds can positively affect poultry health and productivity by providing a natural defense against bacterial attacks (Awuchi, 2019 ![]() ; Ivanova et al., 2024
; Ivanova et al., 2024 ![]() ; Riaz et al., 2023
; Riaz et al., 2023 ![]() ). Herbs, seeds, spices, and plant extracts have been shown to stimulate appetite, improve digestion, and promote the growth of beneficial gut bacteria while reducing pathogenic bacteria (Dahl et al., 2023
). Herbs, seeds, spices, and plant extracts have been shown to stimulate appetite, improve digestion, and promote the growth of beneficial gut bacteria while reducing pathogenic bacteria (Dahl et al., 2023 ![]() ; Frankič et al., 2009
; Frankič et al., 2009 ![]() ). Supplementing poultry diets with plant materials rich in these active substances can enhance immune responses and serve as an effective alternative to antibiotic growth promoters (Ayalew et al., 2022
). Supplementing poultry diets with plant materials rich in these active substances can enhance immune responses and serve as an effective alternative to antibiotic growth promoters (Ayalew et al., 2022 ![]() ; Seidavi et al., 2021
; Seidavi et al., 2021 ![]() ).
).
Plant extracts, known as phytogenic feed additives, are generally free from antibiotic-resistant bacteria and are well-accepted by consumers when used in broiler diets. These plant-derived products are safer, less toxic, and residue-free compared to synthetic antibiotics, making them ideal feed additives (Ayalew et al., 2022 ![]() ; Ivanova et al., 2024
; Ivanova et al., 2024 ![]() ; Upadhaya and Kim, 2017
; Upadhaya and Kim, 2017 ![]() ). Phytogenics enhance animal growth and health by improving digestibility, nutrient absorption, and eliminating gut pathogens. Notably, Chlorella vulgaris, a nutrient-rich microalga, offers essential amino acids, vitamins, and minerals (Karásková et al., 2015
). Phytogenics enhance animal growth and health by improving digestibility, nutrient absorption, and eliminating gut pathogens. Notably, Chlorella vulgaris, a nutrient-rich microalga, offers essential amino acids, vitamins, and minerals (Karásková et al., 2015 ![]() ). It has demonstrated benefits like growth promotion, antioxidant activity, and immunomodulation, making it a promising alternative to antibiotic growth promoters in poultry diets (Abdel-Wareth et al., 2024
). It has demonstrated benefits like growth promotion, antioxidant activity, and immunomodulation, making it a promising alternative to antibiotic growth promoters in poultry diets (Abdel-Wareth et al., 2024 ![]() ).
).
The use of antibiotic growth promoters in poultry feed has raised significant health concerns due to the development of antibiotic-resistant bacteria, necessitating the search for effective alternatives. This study hypothesized that DCP supplementation in broiler diets can improve growth performance, hematological properties, and organ characteristics while effectively controlling E. coli and Salmonella populations, thus serving as a viable alternative to antibiotics. The research aims to evaluate the effects of DCP on broiler growth, health, and intestinal microflora, and to determine the optimal inclusion level of DCP in broiler diets for maximum benefits. The finding could lead to the adoption of dried Chlorella powder as a natural and effective alternative to antibiotic growth promoters in poultry feed, enhancing broiler health and productivity while mitigating antibiotic resistance concerns.
2. Materials and Methods
2.1 Ethical approval
No ethical approval is required for this study.
2.2 Study area and periods
The research work was conducted at Sher-e-Bangla Agricultural University poultry farm, Dhaka, Bangladesh for a period of 28 days from 06th July to 5th August, 2018 (Figure 1).
2.3 Collection of experimental broilers and management
A total of 120 pieces day-old Cobb 500 broiler chicks from Kazi hatchery, Gazipur, Dhaka, were brooded for one week on a basal diet at the university poultry farm. After brooding, 60 chicks were assigned to two dietary treatments of dried Chlorella powder (DCP), and the remaining 60 were assigned to an antibiotic treatment and a control group (Table 1). The study consisted of four treatments, T1, the control group, received a basal diet; T2 received the basal diet supplemented with the antibiotic Doxivet; T3 was fed a basal diet with 0.5% dried Chlorella powder (0.5 kg DCP per 100 kg feed); and T4 received a basal diet with 1% dried Chlorella powder (1 kg DCP per 100 kg feed). Each treatment was replicated three times with 10 birds per replication, totaling 120 broiler chicks.
Table 1. Layout of the experimental design.
2.4 Preparation of experimental house
The experimental room was properly cleaned and washed by using tap water. Ceiling walls and floor were thoroughly cleaned and disinfected by spraying diluted iodophor disinfectant solution (3 ml/liter water). After proper drying, the house was divided into 12 pens of equal size using wood materials and wire net. The height of wire net was 36 cm. A group of 10 birds were randomly allocated to each pen (replication) of the 4 (four) treatments. The stocking density was 1m2/10 birds.
2.5 Experimental diets
Commercial Kazi broiler starter and grower feeds were purchased, containing 19-21% protein as per the feed company's manual. Feeds were supplied four times daily, with ad libitum drinking water twice daily, following the Cobb 500 Manual. Dried Chlorella powder (DCP), imported from the USA, was used in the commercial basal diets (Table 2).
Table 2. Nutritional composition of Chlorella vulgaris (dry matter basis).
2.6 Management procedures
Body weight and feed intake were recorded weekly, and survivability was tracked for each replication up to 28 days. The experiment ran from July 6 to August 5, 2018, with an average temperature of 31.5 °C and 80% relative humidity. Chicks were brooded together for one week, then distributed randomly into pens with 10 chicks per 1m² pen. Due to the hot climate, brooding temperature was adjusted as needed, using an electric bulb for stimulation during the day and providing extra heat only at night when necessary. Daily room temperature and humidity were recorded every six hours. Rice husk was used as litter, stirred daily to prevent gas accumulation and parasite infestation, with fresh litter added as needed. Birds were given feed and water ad libitum, with feeders cleaned weekly and drinkers washed daily. Lighting was provided 24 hours for the first two weeks, then reduced to 22 hours with 2 hours of darkness. Biosecurity measures included vaccination, sanitation, and supplementation with vitamins and electrolytes (Table 3). The south-facing, open-sided broiler shed allowed for easy cross-ventilation, and ventilation was adjusted using polythene screens. Strict sanitation measures, including the use of disinfectant (Virkon), were maintained throughout the experiment.
Table 3. Vaccination schedule for the experimental chicken.
2.7 Study parameters
Weekly live weight, weekly feed consumption and death of chicks to calculate mortality percent. FCR was calculated from the final live weight and total feed consumption per bird in each replication. After slaughter gizzard, liver, spleen, intestine, heart and bursa were measured from each broiler chicken. The dressing yield was calculated for each replication to find out the dressing percentage. The blood sample was analyzed from each replication to measure, Complete blood count (CBC), sugar and cholesterol levels. Feces sample was collected to measure microbial load in the gut.
2.8 Data collection and calculation
The initial and weekly live weights were recorded for each replication to obtain the final live weight per bird. Dressing yield was calculated by subtracting the weight of blood, feathers, head, shank, digestive system, liver, and heart from the live weight. Daily feed consumption was tracked to get weekly and total feed consumption per bird. Mortality was recorded daily up to 28 days of age. For dressing procedures, three birds from each replicate were randomly selected and sacrificed at 28 days. The birds were fasted for 12 hours with water provided ad-libitum, weighed before slaughter, and bled out. Carcasses were washed, eviscerated, and dissected, with the dressing yield calculated by removing specific parts from the live weight. Blood samples were collected and analyzed for glucose, cholesterol, and complete blood count. The average body weight gain was determined by subtracting the initial weight from the final weight,
Body weight gain=Final weight−Initial weight.
Feed intake was calculated as total feed consumption divided by the number of birds in each replication, and the feed conversion ratio (FCR) was calculated as total feed consumption divided by weight gain,
Feed conversion ratio=(Total feed consumption)/(Weight gain)
2.9 Statistical analysis
The data was subjected to statistical analysis by applying one way ANOVA using statistical package for social sciences (SPSS) version 16. Differences between means were tested using Duncan‟s multiple comparison test and significance was set at P<0.05.
3. Results and Discussion
3.1 Production performances of broiler chicken
3.1.1 Final live weight
The relative final live weight (g) of broiler chickens in the dietary groups T1, T2, T3 and T4 were 1610.47±4.67, 1627.47±5.67, 1631.80±5.77 and 1665.13±8.82 respectively. The significantly (P<0.05) highest result was found in T4 (1665.13±8.82) and lowest result was in T1 (1610.47±4.67) group (Table 4). However, final live weight of broiler fed with Chlorella diets increased significantly (P<0.05) compared to that of the control and antibiotic treated groups.
These results are in agreement with those obtained by Kang et al. (2013 ![]() ) who found that several Chlorella-based supplements including DCP, liquid media or CGF added into the diets of broiler chicks enhanced body weight. In addition, these results are in contradictory with those of previous researchers Peiretti and Meineri (2008
) who found that several Chlorella-based supplements including DCP, liquid media or CGF added into the diets of broiler chicks enhanced body weight. In addition, these results are in contradictory with those of previous researchers Peiretti and Meineri (2008 ![]() ), and Takekoshi et al. (2005
), and Takekoshi et al. (2005 ![]() ) reported that dietary Chlorella did not significantly (P>0.05) improve weight gain of chickens compared with the control groups. However, Choi et al. (2017
) reported that dietary Chlorella did not significantly (P>0.05) improve weight gain of chickens compared with the control groups. However, Choi et al. (2017 ![]() ) and Abou-Zeid et al. (2015
) and Abou-Zeid et al. (2015 ![]() ) reported that birds fed dietary Chlorella had beneficial effects on productive performance.
) reported that birds fed dietary Chlorella had beneficial effects on productive performance.
Table 4. Production performance of broiler chicken treated with DCP and antibiotic
3.1.2 Feed consumption (FC)
Chlorella treated T4 (2287.30±8.90) and antibiotic treated T2 (2281.13±10.07) group consumed significantly (P<0.05) lower amount of feed, and T1 control group consumed significantly (P<0.05) higher amount of feed (2338.33±3.17). Antibiotic treated group T2 consumed numerically lower amount of feed compared to T4 group (Table 4).
These results are in contrast with the findings of previous researchers who found that DCP had no effect on feed intake between experimental groups compared with that of control group (Abou-Zeid et al., 2015 ![]() ; Kang et al., 2013
; Kang et al., 2013 ![]() ). Finding of this experiment of FC are in agreement with those of previous researchers who recorded that dietary micro algae spirulina significantly (P<0.05) improved Feed consumption (FC) of broiler chickens in different inclusion levels (Hassan et al., 2022
). Finding of this experiment of FC are in agreement with those of previous researchers who recorded that dietary micro algae spirulina significantly (P<0.05) improved Feed consumption (FC) of broiler chickens in different inclusion levels (Hassan et al., 2022 ![]() ; Mirzaie et al., 2018
; Mirzaie et al., 2018 ![]() ).
).
3.1.3 Feed Conversion Ratio (FCR)
Feed conversion ratio (FCR) in different groups were significantly (P<0.05) different and the highest FCR was in T1 (1.45±0.00) group and lowest FCR was in T4 (1.37±0.01) group (Table 4). These results are in agreement with those of previous researchers Khalilnia et al. (2023 ![]() ) and El-Shall et al. (2023
) and El-Shall et al. (2023 ![]() ) who reported that dietary Chlorella significantly (P<0.05) improved feed efficiency of broiler chickens compared with the control groups.
) who reported that dietary Chlorella significantly (P<0.05) improved feed efficiency of broiler chickens compared with the control groups.
The improvement of feed conversion ratio in Chlorella and prebiotic treated broilers could be related to better equilibrium in the intestinal flora (Bedford, 2000 ![]() ). These results are in contradictory with those of previous researchers Kang et al. (2013
). These results are in contradictory with those of previous researchers Kang et al. (2013 ![]() ) and Oh et al. (2015
) and Oh et al. (2015 ![]() ) who showed that there were no significant effects on feed efficiency between the Chlorella treated and control groups.
) who showed that there were no significant effects on feed efficiency between the Chlorella treated and control groups.
3.1.4 Dressing percentage
The T4 (71.39±0.54) and T3 (71.09±0.45) DCP supplemented group had greater (P<0.05) dressing percentage compared with the control (67.51±0.29) group (Table 4). These findings are in accordance with the findings of El-Deek et al. (2011 ![]() ) who showed that thermal or enzymatic treatments, using different levels of algae in broiler finisher diets had significant effect on dressing percentages (ranged between 73.1 to 73.8%) at 39 days of age. These results are contradictory with the Abdelnour et al. (2019
) who showed that thermal or enzymatic treatments, using different levels of algae in broiler finisher diets had significant effect on dressing percentages (ranged between 73.1 to 73.8%) at 39 days of age. These results are contradictory with the Abdelnour et al. (2019 ![]() ) who recorded non-significant (P>0.05) effects of dietary Chlorella supplementation on dressing percentages as compared to control group.
) who recorded non-significant (P>0.05) effects of dietary Chlorella supplementation on dressing percentages as compared to control group.
3.1.5 Weekly body weight gain
The mean body weight gains (g) of broiler chicks at the end of 4th week in different groups were 652.50±13.61, 726.83±3.33, 659.03±1.35 and 673.50±.00 respectively (Table 5). At the end of 1st week the body weight gain in different groups were non-significant (P>0.05). T2 group had the higher body weight gain than other groups. At the end of 4th week the body weight gain in different groups were significantly different (P<0.05). T2 group had the higher body weight gain (726.83±3.33) than other group. According to Choi et al. (2017 ![]() ) broilers fed the PC2 treatment group (1.0% EFL with Chlorella) (P< 0.05) exhibited higher BWG than in the primary NC treatment group.
) broilers fed the PC2 treatment group (1.0% EFL with Chlorella) (P< 0.05) exhibited higher BWG than in the primary NC treatment group.
Table 5. Effects of feeding different level of DCP and antibiotic on body weight gain (BWG) (g/bird) of broiler chickens at different weeks.
3.1.6 Weekly feed consumption (FC)
The mean FC (g) of broiler chicks at the end of 4th week in different groups were 1004.80±5.57, 1000.90±15.09, 991.23±5.12 and 1008.00±4.368 correspondingly (Figure 2). The overall mean FC of different groups showed that there were no significant (P>0.05) difference between different treatment groups. These findings are in accordance with Takekoshi et al. (2005 ![]() ) who indicated that dietary supplementation of Chlorella (Chlorella pyrenoidosa) did not affect the feed intake of mice. An et al. (2016
) who indicated that dietary supplementation of Chlorella (Chlorella pyrenoidosa) did not affect the feed intake of mice. An et al. (2016 ![]() ) also showed Chicks fed diets with 0.15 or 0.5 % DCP had no effect on feed intake between experimental groups compared with that of control group.
) also showed Chicks fed diets with 0.15 or 0.5 % DCP had no effect on feed intake between experimental groups compared with that of control group.

3.1.7 Weekly feed conversion ratio (FCR)
The mean body FCR of broiler chicks at the end of 4th week in different groups were 1.55±0.03, 1.36±0.01, 1.52±0.01 and 1.49±0.02 respectively. The overall mean FCR of different groups showed that there was a significant (P<0.05) difference in groups. T2 showed the lowest FCR compared to control and other treatment groups (Table 6). These findings are in line with the findings of Abou-Zeid et al. (2015 ![]() ) dietary treatments improved feed conversion ratio compared to the birds fed control diet during starter, finisher and the whole experimental periods. In contrast these findings are opposite to the result of Oh et al. (2015
) dietary treatments improved feed conversion ratio compared to the birds fed control diet during starter, finisher and the whole experimental periods. In contrast these findings are opposite to the result of Oh et al. (2015 ![]() ) who reported that there were no significant effect on feed efficiency between the Chlorella treated and control groups. Kang et al. (2013
) who reported that there were no significant effect on feed efficiency between the Chlorella treated and control groups. Kang et al. (2013 ![]() ) also reported that several Chlorella-based supplements including DCP, liquid media or CGF added into the diets of broiler chicks did not affect feed conversion ratio.
) also reported that several Chlorella-based supplements including DCP, liquid media or CGF added into the diets of broiler chicks did not affect feed conversion ratio.
Table 6. The effects of feeding DCP and antibiotics on FCR of broiler chickens at different weeks.
3.2 Blood glucose and cholesterol
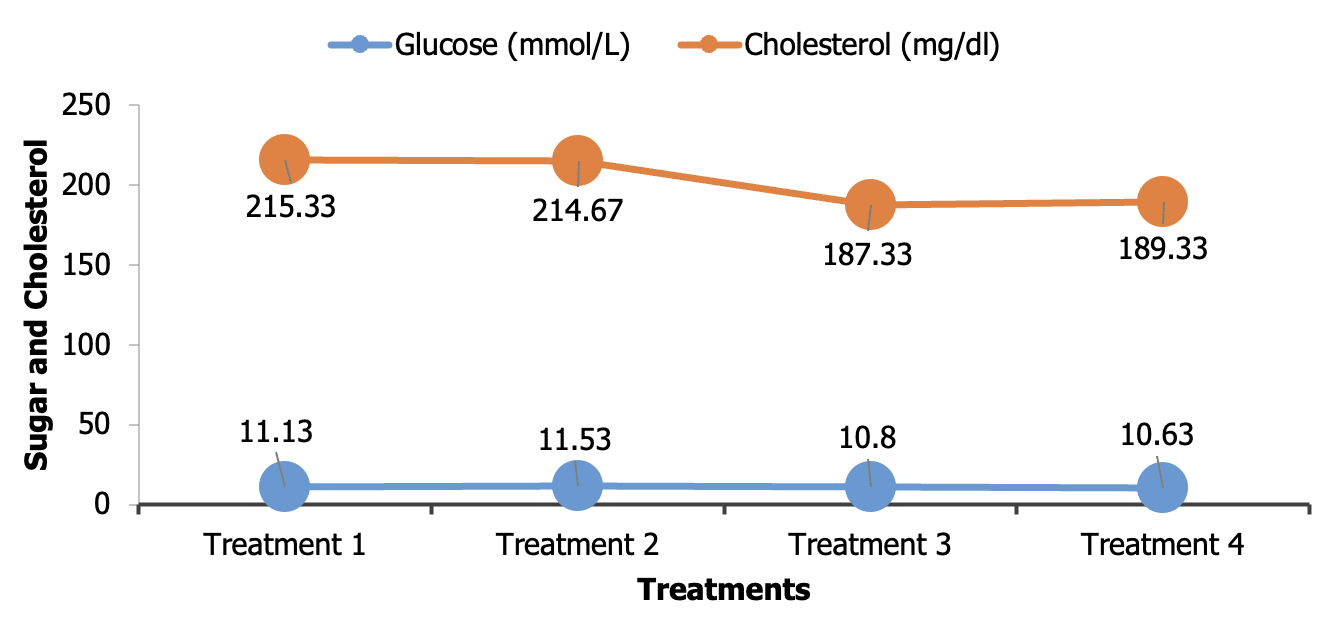
Although the highest amount (11.53±0.54 m mol/L) of plasma glucose was found in T2 but this was not statistically different (P>0.05) with control and other groups (Figure 3). The results of the present study are compatible with those observed by Kotrbáček et al. (2015 ![]() ) and An et al. (2016
) and An et al. (2016 ![]() ) observed incorporation of dietary Chlorella in broilers diet had no significant (P>0.05) effect on serum glucose level of broiler chicken. The increase in plasma glucose concentration of hens fed dietary Chlorella may be attributed to its excellent nutritional profile and high carotenoid content. Total cholesterol concentration (mg/dl) in the serum of T1, T2, T3 and T4 groups were 215.33±33.01, 214.67±10.17, 187.33±10.41 and 189.33±14.11 respectively. Statistical analysis revealed no significant (P>0.05) difference among the group (Figure 3). However the cholesterol level was lower in T3 fed group (187.33±10.414) numerically but not statistically. Similar results had also been observed by Kotrbáček et al. (2015
) observed incorporation of dietary Chlorella in broilers diet had no significant (P>0.05) effect on serum glucose level of broiler chicken. The increase in plasma glucose concentration of hens fed dietary Chlorella may be attributed to its excellent nutritional profile and high carotenoid content. Total cholesterol concentration (mg/dl) in the serum of T1, T2, T3 and T4 groups were 215.33±33.01, 214.67±10.17, 187.33±10.41 and 189.33±14.11 respectively. Statistical analysis revealed no significant (P>0.05) difference among the group (Figure 3). However the cholesterol level was lower in T3 fed group (187.33±10.414) numerically but not statistically. Similar results had also been observed by Kotrbáček et al. (2015 ![]() ), who reported that dietary DCP did not affect the concentration of plasma triacylglycerol and cholesterol in laying hens. Study of Panaite et al. (2023
), who reported that dietary DCP did not affect the concentration of plasma triacylglycerol and cholesterol in laying hens. Study of Panaite et al. (2023 ![]() ) had shown contradictory result that Chlorella reduces cholesterol and increases the omega-3 content of eggs.
) had shown contradictory result that Chlorella reduces cholesterol and increases the omega-3 content of eggs.
3.3 Relative giblet and intestine weight
The relative weight of liver (g) of broiler chicks in the dietary groups T1, T2, T3 and T4 were 37.33±0.16, 38.87±0.32, 40.13±0.91 and 38.50±1.53 respectively. The highest results were in T3 and lowest was in T1 group. However, there were no significant (P>0.05) difference in the relative weight of liver between the groups (Table 7). This results are in line with the findings of An et al. (2016 ![]() ) who reported that dietary Chlorella did not affect relative organ weights including liver, spleen, bursa of Fabricius and abdominal fat. Research of El-Deek et al. (2011
) who reported that dietary Chlorella did not affect relative organ weights including liver, spleen, bursa of Fabricius and abdominal fat. Research of El-Deek et al. (2011 ![]() ) also accomplished that using different levels of algae in broiler finisher diets had insignificant (P>0.05) effect on gizzard weights. The comparative weight of heart (g) of broiler chicks in the dietary group T1, T2, T3 and T4 were 9.33±0.60, 9.50±0.50, 10.50±0.00 and 9.17±0.73 correspondingly. The qualified weight of hearts of different groups showed that there were no significant (P>0.05) differences between the groups (Table 10). Abdelnour et al. (2019
) also accomplished that using different levels of algae in broiler finisher diets had insignificant (P>0.05) effect on gizzard weights. The comparative weight of heart (g) of broiler chicks in the dietary group T1, T2, T3 and T4 were 9.33±0.60, 9.50±0.50, 10.50±0.00 and 9.17±0.73 correspondingly. The qualified weight of hearts of different groups showed that there were no significant (P>0.05) differences between the groups (Table 10). Abdelnour et al. (2019 ![]() ) also reported that supplementing the rabbit diets with CLV did not induce significant differences (P>0.05) in giblets, heart, kidney, lung, and liver as compared to the control animals. It means that fenugreek infusion having antimicrobial and antibiotics like properties have no influence on either increasing or decreasing the relative weights of giblet.
) also reported that supplementing the rabbit diets with CLV did not induce significant differences (P>0.05) in giblets, heart, kidney, lung, and liver as compared to the control animals. It means that fenugreek infusion having antimicrobial and antibiotics like properties have no influence on either increasing or decreasing the relative weights of giblet.
The results of different groups showed that there were no significant (P>0.05) differences between the groups and the values were ranged from 84.67±0.88 to 92.67±4.37 (Table 7). In the study of An et al. (2016 ![]() )), they showed Chlorella had no impact on visceral organs (liver, heart, gizzard, and intestines) of broiler chicks.
)), they showed Chlorella had no impact on visceral organs (liver, heart, gizzard, and intestines) of broiler chicks.
Table 7. Effect of dietary supplementation of DCP on liver, gizzard, intestine and heart weight of different treatments.
3.4 Immune organs (spleen and bursa)
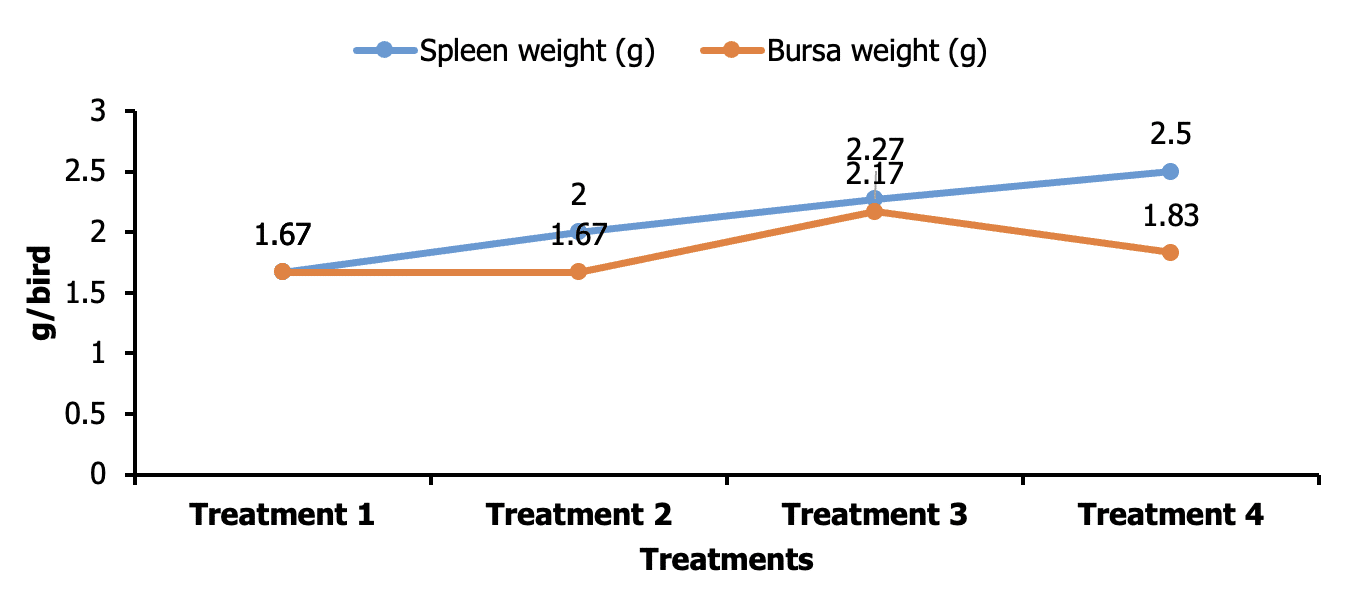
The comparative weight of spleen (g) of broiler chicks in the dietary group T1, T2, T3 and T4 were 1.67±0.44, 2.00±0.50, 2.27±0.27 and 2.50±0.00 respectively. The highest value was T4 (2.50±0.00) and lowest value was T1 (1.67±0.44). On the other hand, the relative weight of spleen of different groups showed that there were no significant (P>0.05) difference. The weight of bursa was higher in T3 group (2.17±0.333) compared to the other group whose values were T1 (1.67±0.17), T2 (1.67±0.17) and T4 (1.83±0.17) correspondingly. But these values are not significantly differing among the treatments (Figure 4).
An et al. (2016 ![]() ) reported that dietary Chlorella did not affect relative organ weights including spleen, bursa of Fabricius and abdominal fat. Schiavone et al. (2007
) reported that dietary Chlorella did not affect relative organ weights including spleen, bursa of Fabricius and abdominal fat. Schiavone et al. (2007 ![]() ) also found that using of 5g algae/kg feed insignificantly affected on the slaughter characteristics of the Muscovy ducks.
) also found that using of 5g algae/kg feed insignificantly affected on the slaughter characteristics of the Muscovy ducks.
3.5 Hematological parameters
Concerning the treatment effect on blood constituents, the results indicated no significant differences due to supplementation of DCP, except Hemoglobin and RBC which were significantly affected (P>0.05). Birds fed diets supplemented with Chlorella (at levels of 0.5% and 1%) diet had higher values of Hemoglobin and RBC but in case of control group these trends were lower than Chlorella treated groups (Table 8).
Table 8. Effect of supplementation of DCP to broiler diets on blood parameters.
These results align with the earlier findings of An et al. (2010 ![]() ), who reported increased levels of total protein, albumin, glucose, and interferon-γ in the blood serum of mice fed with a hot water extract of Chlorella.
), who reported increased levels of total protein, albumin, glucose, and interferon-γ in the blood serum of mice fed with a hot water extract of Chlorella.
3.6 Intestinal microflora
E. coli count was significantly (P<0.05) decreased in birds fed 0.5%, 1% Chlorella and antibiotic (11.00±0.30, 11.23±0.44 and 11.68±0.34 respectively) than the control birds (15.58±0.87). Salmonella sp. count was also significantly (P<0.05) decreased in birds fed 0.5%, 1% DCP and antibiotic (5.70±1.55, 4.66±1.67 and 9.03±1.33 respectively) than the control birds (14.46±1.25). Lactobacillus count was significantly (P<0.05) increased in birds fed 0.5% and 1% Chlorella. The highest number of lactobacillus was counted in T4 group (19.76±0.38) and the lowest in T1 group (11.70±0.33) (Table 9).
Table 9. Bacterial colony count in DCP experiment in broiler chicken.
These results of the experiment are in accordance with the earlier findings of Janczyk et al. (2009 ![]() ) who reported that feeding Chlorella vulgaris significantly increased the lactobacilli diversity in crop and ceca of laying hens with a stronger effect on the cecal bacterial population. Nigussie et al. (2021
) who reported that feeding Chlorella vulgaris significantly increased the lactobacilli diversity in crop and ceca of laying hens with a stronger effect on the cecal bacterial population. Nigussie et al. (2021 ![]() )reported that methanol extracts of C. vulgaris lowered E. coli and Salmonella. However, the population of cecal coliform bacteria in ducks fed diet with 2,000 mg/kg fermented C. vulgaris tended to be lower compared with their control-diet fed counterparts (linear effect at P=0.064), indicating that C. vulgaris may have a positive effect on improving cecal microflora (Oh et al., 2015
)reported that methanol extracts of C. vulgaris lowered E. coli and Salmonella. However, the population of cecal coliform bacteria in ducks fed diet with 2,000 mg/kg fermented C. vulgaris tended to be lower compared with their control-diet fed counterparts (linear effect at P=0.064), indicating that C. vulgaris may have a positive effect on improving cecal microflora (Oh et al., 2015 ![]() ).
).
3.7 Antiviral activity
Concerning the treatment effect on HI titre the results indicated significant (P<0.05) differences due to supplementation of DCP. Remarkably better titres of ND were achieved in blood at day 15 (5.56±0.24) and day 29 (6.89±0.26) in the T4 treatments compared to control group (Table 10). It is reported that either DCP or CGF improved immune functions in rodents and chickens (An et al., 2016 ![]() ; Kang et al., 2013
; Kang et al., 2013 ![]() ). Kang et al. (2013
). Kang et al. (2013 ![]() ) reported that dietary supplementation of Chlorella significantly (P< 0.05) increased the plasma IgA, IgM and IgG concentration of chicks compared with AGP and control. In contrast, these results are contradictory with the earlier findings of An et al. (2016
) reported that dietary supplementation of Chlorella significantly (P< 0.05) increased the plasma IgA, IgM and IgG concentration of chicks compared with AGP and control. In contrast, these results are contradictory with the earlier findings of An et al. (2016 ![]() ) who found that the antibody titers against NDV and IBV in chicks were not affected by DCP and CGF. Immurella, a polysaccharide compound in the Chlorella cells, is also an important factor to enhance the immune response of broilers fed Chlorella-supplemental diets (Pugh et al., 2001
) who found that the antibody titers against NDV and IBV in chicks were not affected by DCP and CGF. Immurella, a polysaccharide compound in the Chlorella cells, is also an important factor to enhance the immune response of broilers fed Chlorella-supplemental diets (Pugh et al., 2001 ![]() ).
).
Table 10. Effect of DCP on pre-vaccination ND HI titre in broiler chicken.
4. Conclusions
The current study demonstrated that broilers fed with dried Chlorella powder, particularly at one percent inclusion, showed significant improvements in body weight, feed conversion ratio, hemoglobin, and red blood cell counts compared to control and antibiotic groups. Additionally, Chlorella supplementation resulted in lower cholesterol levels, higher Lactobacillus counts, and enhanced immune response, indicating its potential as a viable alternative to antibiotics in broiler diets. These findings suggest that Chlorella can effectively enhance growth performance and health in broilers.
Acknowledgements
The authors would like to thank Department of Poultry Science, Sher-e-Bangla Agricultural University for logistic and laboratory facilities provided during the investigation. The first author sincerely acknowledges the financial grant support from Ministry of Science and Technology, People’s Republic of Bangladesh as National Science and Technology (NST) fellowship.
Data availability statement
The data generated from this study might be shared with a valid request from the corresponding author.
Informed consent statement
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
Conceptualization: NAM, MAHB and KBMSI; Data collection: NAM and SA; Data analysis: NAM, PB and MZR; Figure preparation: NAM PB and MZR. All authors critically reviewed the manuscript and agreed to submit final version of the manuscript. All authors critically reviewed the manuscript and agreed to submit final version of the manuscript.



