Microalgae are widely regarded as the optimal first feed for mollusks, shrimp, and fish in the aquaculture industry due to their small size and rich nutritional profile (Brown and Blackburn, 2013 ![]() ; Ma and Hu, 2024
; Ma and Hu, 2024 ![]() ; Gao et al., 2024
; Gao et al., 2024 ![]() ). These microscopic organisms are packed with essential nutrients, including proteins, fatty acids, vitamins, and minerals, making them an ideal food source for the early life stages of aquatic species (Bhattacharjya et al., 2024
). These microscopic organisms are packed with essential nutrients, including proteins, fatty acids, vitamins, and minerals, making them an ideal food source for the early life stages of aquatic species (Bhattacharjya et al., 2024 ![]() ; Dineshbabu et al., 2019
; Dineshbabu et al., 2019 ![]() ). Microalgae provide a balanced diet, promotes healthy growth and development, ensuring higher survival rates and improved overall performance of larvae, broods, herbivorous aquatic organisms thus playing a crucial role in supporting sustainable and efficient aquaculture and hatchery operations (Brown and Blackburn, 2013
). Microalgae provide a balanced diet, promotes healthy growth and development, ensuring higher survival rates and improved overall performance of larvae, broods, herbivorous aquatic organisms thus playing a crucial role in supporting sustainable and efficient aquaculture and hatchery operations (Brown and Blackburn, 2013 ![]() ; Ma and Hu, 2024
; Ma and Hu, 2024 ![]() ; Gao et al., 2024
; Gao et al., 2024 ![]() ). Microalgae culture has become one of the prime considerations and most important nutrition options for the hatchery development of fish, shellfish and other invertebrates due to their dependency as staple food supply during early life stage (Gao et al., 2024
). Microalgae culture has become one of the prime considerations and most important nutrition options for the hatchery development of fish, shellfish and other invertebrates due to their dependency as staple food supply during early life stage (Gao et al., 2024 ![]() ; Rofidi, 2017
; Rofidi, 2017 ![]() ). Microalgal biomass has recently gained interest as a food ingredient for both humans and animals, as well as a feedstock for biofuels and bulk chemicals for nutraceuticals, cosmeceuticals and pharmaceuticals (Jbari et al., 2020
). Microalgal biomass has recently gained interest as a food ingredient for both humans and animals, as well as a feedstock for biofuels and bulk chemicals for nutraceuticals, cosmeceuticals and pharmaceuticals (Jbari et al., 2020 ![]() ; Mahata et al., 2022
; Mahata et al., 2022 ![]() ; Tokushima et al., 2016
; Tokushima et al., 2016 ![]() ). Cultivating this alga on a large scale using pure chemical media is cost-extensive, labor-intensive and has limited economic feasibility (de Carvalho et al., 2019
). Cultivating this alga on a large scale using pure chemical media is cost-extensive, labor-intensive and has limited economic feasibility (de Carvalho et al., 2019 ![]() ; Oostlander et al., 2020
; Oostlander et al., 2020 ![]() ). The high cost incurred in mass culture of algae remains a significant barrier to the growth of the aquaculture industry globally (Oostlander et al., 2020
). The high cost incurred in mass culture of algae remains a significant barrier to the growth of the aquaculture industry globally (Oostlander et al., 2020 ![]() ). Therefore many researchers has focused on identifying more affordable cost-effective nutrient sources alternative to pure media including commercial fertilizers, industrial grade inorganic salts, and soil extract (Jbari et al., 2020
). Therefore many researchers has focused on identifying more affordable cost-effective nutrient sources alternative to pure media including commercial fertilizers, industrial grade inorganic salts, and soil extract (Jbari et al., 2020 ![]() ; Qayyum et al., 2020
; Qayyum et al., 2020 ![]() ; Tahiri et al., 2024
; Tahiri et al., 2024 ![]() ; Ribeiro et al., 2020
; Ribeiro et al., 2020 ![]() ).
).
The utilization of commercially available low-cost local ingredients for supplying algae in local hatchery and aquaculture industry may open a new research opportunities alternative to the use of pure laboratory grade media. However, the growth of microalgae is dependent on the supply of nutrients such as phosphates, nitrates and silicates, and specific vitamins, proteins, and other trace elements (Croft et al., 2006 ![]() ; de Carvalho et al., 2019
; de Carvalho et al., 2019 ![]() ). The level of fatty acids and biochemical composition of algae may change with the concentrations of nutrients (Dineshbabu et al., 2019
). The level of fatty acids and biochemical composition of algae may change with the concentrations of nutrients (Dineshbabu et al., 2019 ![]() ). Many studies described that algal species required different combinations of vitamins, particularly vitamin B12 (cobalamin), vitamin B1 (thiamine), and vitamin B7 (biotin) (Croft et al., 2006
). Many studies described that algal species required different combinations of vitamins, particularly vitamin B12 (cobalamin), vitamin B1 (thiamine), and vitamin B7 (biotin) (Croft et al., 2006 ![]() ; Helliwell, 2017
; Helliwell, 2017 ![]() ). Vitamin B was suggested to perform different functions in algae including growth promoting factor, aiding in carbon metabolism, acting as a cofactor for enzymes (Croft et al., 2006
). Vitamin B was suggested to perform different functions in algae including growth promoting factor, aiding in carbon metabolism, acting as a cofactor for enzymes (Croft et al., 2006 ![]() ; Helliwell, 2017
; Helliwell, 2017 ![]() ). The concentration of vitamin B in the natural sea or freshwater is not enough to support the culture of microalgae, thus these vitamins should be supplemented during culture media preparation (Croft et al., 2006
). The concentration of vitamin B in the natural sea or freshwater is not enough to support the culture of microalgae, thus these vitamins should be supplemented during culture media preparation (Croft et al., 2006 ![]() ).
).
Unicellular algae Chaetoceros gracilis is a widely used diatom as a live food in aquaculture and hatcheries due to its rich nutritional profile which support the growth and health of penaeid shrimp, mollusks and fishes (Gao et al., 2024 ![]() ; Ma and Hu 2024
; Ma and Hu 2024 ![]() ). This species can be a potential live feed for the broods and larvae of hard clam and oyster. It is particularly important for feeding larvae of mollusks, crustaceans, and some fish species, enhancing survival rates, promoting proper development, and improving overall aquaculture productivity (Tachihana et al., 2023
). This species can be a potential live feed for the broods and larvae of hard clam and oyster. It is particularly important for feeding larvae of mollusks, crustaceans, and some fish species, enhancing survival rates, promoting proper development, and improving overall aquaculture productivity (Tachihana et al., 2023 ![]() ). This species also holds potential for biofuel production due to its high lipid content, which can be converted into biodiesel (Perez et al., 2017
). This species also holds potential for biofuel production due to its high lipid content, which can be converted into biodiesel (Perez et al., 2017 ![]() ). As a diatom, it has the ability to efficiently photosynthesize and accumulate energy-rich compounds, including fatty acids and fucoxanthin production, making it a promising candidate for renewable energy sources (Perez et al., 2017
). As a diatom, it has the ability to efficiently photosynthesize and accumulate energy-rich compounds, including fatty acids and fucoxanthin production, making it a promising candidate for renewable energy sources (Perez et al., 2017 ![]() ; Tokushima et al., 2016
; Tokushima et al., 2016 ![]() ).
).
Laboratory culture of micro-algae is labor-intensive and requires significant resources like synthetic medium, stored seawater, lighting, aeration and sometimes temperature control (Ahmad et al., 2022 ![]() ). Using synthetic media for algae culture presents several disadvantages, including high production costs due to the need for expensive nutrients and chemicals, as well as the potential for nutrient imbalances that can hinder growth (Ahmad et al., 2022
). Using synthetic media for algae culture presents several disadvantages, including high production costs due to the need for expensive nutrients and chemicals, as well as the potential for nutrient imbalances that can hinder growth (Ahmad et al., 2022 ![]() ; Chakraborty et al., 2023
; Chakraborty et al., 2023 ![]() ). Additionally, synthetic media often limit biodiversity by favoring specific strains, which reduces adaptability to environmental changes (Chakraborty et al., 2023
). Additionally, synthetic media often limit biodiversity by favoring specific strains, which reduces adaptability to environmental changes (Chakraborty et al., 2023 ![]() ). However, these challenges open up opportunities for exploring alternative culture media, such as locally sourced ingredients or natural materials, which can be more cost-effective and sustainable. Utilizing organic waste products or agricultural byproducts as nutrient sources not only reduces reliance on synthetic components but also promotes a circular economy in algae cultivation, potentially enhancing growth and productivity while minimizing environmental impact (Ahmad et al., 2022
). However, these challenges open up opportunities for exploring alternative culture media, such as locally sourced ingredients or natural materials, which can be more cost-effective and sustainable. Utilizing organic waste products or agricultural byproducts as nutrient sources not only reduces reliance on synthetic components but also promotes a circular economy in algae cultivation, potentially enhancing growth and productivity while minimizing environmental impact (Ahmad et al., 2022 ![]() ; Chakraborty et al., 2023
; Chakraborty et al., 2023 ![]() ; Jbari et al., 2020
; Jbari et al., 2020 ![]() ). The potential of mass culture C. gracilis using commercial agriculture fertilizers, zeolitic products were studied (Jbari et al., 2020
). The potential of mass culture C. gracilis using commercial agriculture fertilizers, zeolitic products were studied (Jbari et al., 2020 ![]() ; López-Ruiz et al., 1995
; López-Ruiz et al., 1995 ![]() ). However, the effect of different combination of fertilizers including compost, NPK and vitamin supplement was not studied before.
). However, the effect of different combination of fertilizers including compost, NPK and vitamin supplement was not studied before.
In this study, we endeavor to develop a culture media for C. gracilis with the ingredients which would resemble F/2 media, but utilizing locally available low-cost fertilizers. In the first phase, the cell density (cells per ml) of C. gracilis in several potential combinations of fertilizers was evaluated to identify the best performing fertilizers recipe. In the second phase, the effect of addition of vitamin supplements, particularly vitamin B, on the cell density of C. gracilis with the best performing fertilizer combination was investigated. Finally, the potentiality of continuous culture of C. gracilis was evaluated in 300 L tank.
2. Materials and Methods
2.1 Ethical approval
No ethical approval is required for this study.
2.2 Study area and location
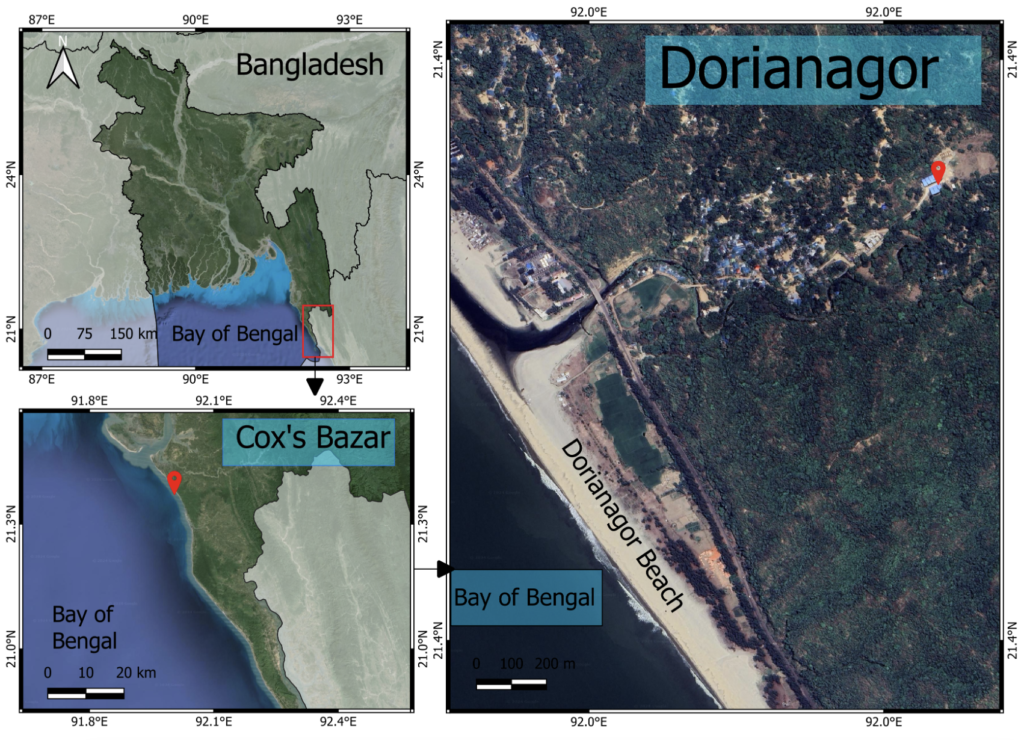
A modified culture media was formulated with the locally available ingredients to support the algal growth in the hatchery facility for brood clam conditioning. The aim was to develop a culture media mimicking the chemical composition of Guillard F/2 media (Guillard, 1975 ![]() ). The experiment was conducted at the marine hatchery building of Coastal Biodiversity, Marine Fisheries and Wildlife Research Centre, Chattogram Veterinary and Animal Sciences University (CVASU), Dorianagar, Cox’s Bazar, Bangladesh (Figure 1) which providing an ideal environment for the cultivation of marine microalgae due to the availability of fresh sea water, filtration and aeration facility.
). The experiment was conducted at the marine hatchery building of Coastal Biodiversity, Marine Fisheries and Wildlife Research Centre, Chattogram Veterinary and Animal Sciences University (CVASU), Dorianagar, Cox’s Bazar, Bangladesh (Figure 1) which providing an ideal environment for the cultivation of marine microalgae due to the availability of fresh sea water, filtration and aeration facility.

2.3 Selection of ingredients for modified fertilizer media
To create the alternative culture media, components were meticulously chosen to replicate the chemical composition of the well-established F/2 media, as described by Guillard (1975 ![]() ). These components were purchased from local agricultural equipment suppliers, promoting the use of readily available resources and reducing dependence on specialized suppliers. The quantities of these locally available ingredients were precisely measured to match the formulations of the F/2 media, ensuring the nutritional adequacy required for optimal algal growth.
). These components were purchased from local agricultural equipment suppliers, promoting the use of readily available resources and reducing dependence on specialized suppliers. The quantities of these locally available ingredients were precisely measured to match the formulations of the F/2 media, ensuring the nutritional adequacy required for optimal algal growth.
Table 1. Locally available ingredients with the local/ trade name and amount for primary stock solution (PSS) and working stock solution (WSS) used in experiments.
2.4 Research design
There were two separate experiments conducted to find the best combinations of local ingredients to develop a mass culture media for C. gracilis in the hatchery facility. The first experiment was conducted to determine effective composition of fertilizer in four combinations as four treatments (T1, T2, T3 and T4) with three replications of each (Table 2). Mineral mix+EDTA and Silicate gel were common across all treatments with varying fertilizer combinations. The treatment which showed the highest cell density was selected as control and common fertilizer combination for experiment 02 for determining the effect of locally available vitamin addition in three treatments (V0-control, V1-multivitamin-Zinc B, V2-Neobion) with three replications of each (Table 2). In 5ml multivitamin-Zinc B, the ingredients were Zinc sulfate monohydrate USP equivalent to elemental Zinc 10 mg; Thiamin hydrochloride USP 5mg; Riboflavin-5 phosphate sodium USP equivalent to riboflavin 2 mg; Pyridoxine hydrochloride USP 2 mg; Nicotinamide USP 20 mg. Neobion contained vitamin B1, B6 and B12.
Table 2. Experimental design to evaluate the effect of fertilizers and vitamins on the cell density (cells/ml) of Chaetoceros gracilis in two experimental setup.
2.5 Micro-algal sample collection and stock preparation
A 200 ml pure stock of C. gracilis was carefully collected from the Department of Aquaculture, CVASU, ensuring the selection of a healthy and robust strain. The preparation of the primary stock and working stock solutions followed the formulation protocols established by Uddin and Zafar (2007 ![]() ). C. gracilis was cultured in 1L conical flask with filtered (1 µm filter) seawater (28 ppt) for 05 days with 24h artificial light and aeration facility within a thick layer of white polythene to avoid contamination and loss of light intensity. Once prepared, the primary stock solutions were stored in a refrigerator to preserve their stability and biological activity, minimizing degradation over time.
). C. gracilis was cultured in 1L conical flask with filtered (1 µm filter) seawater (28 ppt) for 05 days with 24h artificial light and aeration facility within a thick layer of white polythene to avoid contamination and loss of light intensity. Once prepared, the primary stock solutions were stored in a refrigerator to preserve their stability and biological activity, minimizing degradation over time.
2.6 Experimental setup
Before conducting the experiment, the growth curve of C. gracilis was established to determine the durations of the exponential growth and death phases using various media compositions, including Urea + TSP, mineral mix + EDTA, silicate gel, commercial NPK, and commercial compost in 1-liter conical flasks under hatchery conditions with 24-hour artificial light and aeration. Vitamins were not added for this purpose.
2.6.1 Effect of fertilizer combinations experiment
In the experiment 1, for finding out the effective modified media for C. gracilis culture using locally available ingredients, 20% primary stock inoculation plus 80% filtered (1 µm filter) seawater (28 ppt) and modified fertilizer media was used. For preparing 1L working solution, 1 ml of modified fertilizer media (according to treatments) was poured in 1 L conical flask, which was mixed with 100 ml filtered seawater. Then, 200 ml (20% of 1 L) stock solution was added followed by addition of filtered seawater to reach a final volume of 1000 ml. Following similar procedure, working solution for four treatments (Table 2) were set triplicate. The initial cell density was determined through counting algal cells found within ten cells of the sedge-wick rafter counting chamber using the following formula: After day three, the final cell density was measured using sedge-wick rafter counting cell under a compound microscope (Optika B-190TB, Italy) at 20X magnification connected to a digital camera (CB 10) and Computer. The images was taken for each squares seen under microscope and was counted using Image J software. The cell density was measured using the following formula by Hotzel and Croome (1999),
C = (N x 1000 mm3) / (L x D x W x S)
Where, N = number of cells/units counted, L = length of each traverse (mm), W = width of traverse (mm), D = depth of a field (Sedgwick-Rafter chamber depth) (mm), S = number of traverses counted.
2.6.2 Effect of vitamin experiment
In experiment 2, for investigating the effect of addition of vitamins on the cell density of C. gracilis, three treatments were set (V0, V1 and V2) using the best effective modified media composition found after experiment 01. Vitamins were not added in control (V0). Multivitamin-Zinc B, and Neobion was added with modified media in treatment V1 and V2, respectively. Working media was prepared similar way to experiment 1 with the addition of 1ml vitamins. After three days of culture, cell density was measured and compared among treatments.
2.7 Continuous mass culture of C. gracilis
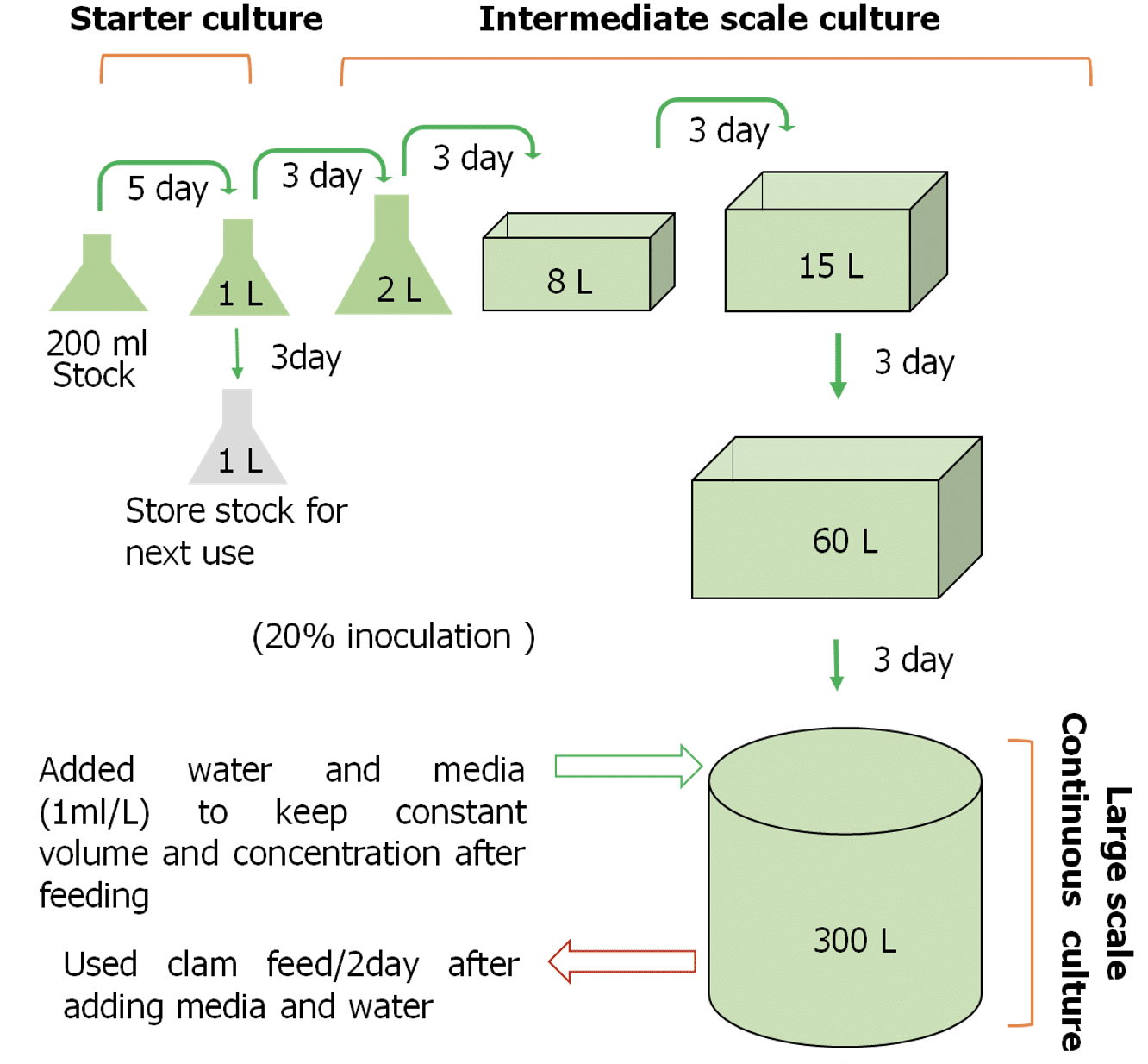
For mass continuous culture, the best performed media combination which was found after experiment 02 was used for continuous plankton production in the hatchery facility in 300 L tank. The culture volume was increased in a step-wise volume increase manner from 200ml to 1, 2, 8, 15, 60, and 300L (Figure 2). The final volume achieved at each culture stage was used as inoculation volume of cell sample (20% of 1 L = 200 ml) for the next culture volume. From starter to intermediate culture, five days were allowed to grow the algae to reach at peak cell density. However from intermediate to mass culture stages, culture was done for three days (Figure 2). After reaching 300 L volume, 180 L was supplied for clam feeding in the every two days interval. Immediately after removal of algae for clam feeding, filtered seawater and modified media (containing best performing fertilizer and vitamin combination) was supplemented to reach 300 L final culture volume and maintaining the original level of enrichment following (Lavens and Sorgeloos, 1996 ![]() ). The cell density (cell/ml) up to eight days were observed to check whether continuous culture of C. gracilis is possible in hatchery facility.
). The cell density (cell/ml) up to eight days were observed to check whether continuous culture of C. gracilis is possible in hatchery facility.
2.8 Data analysis
For statistical analysis, one-way ANOVA and Tukey post hoc tests were conducted using IBM SPSS 26.0 to determine significant variations in cell density among the different culture media ingredient compositions and among the vitamin treatments. Tukey’s post-hoc test was done to find the homogenous subsets with the level of significance 0.05%.
3. Results
3.1 Growth curve of Chaetoceros gracilis
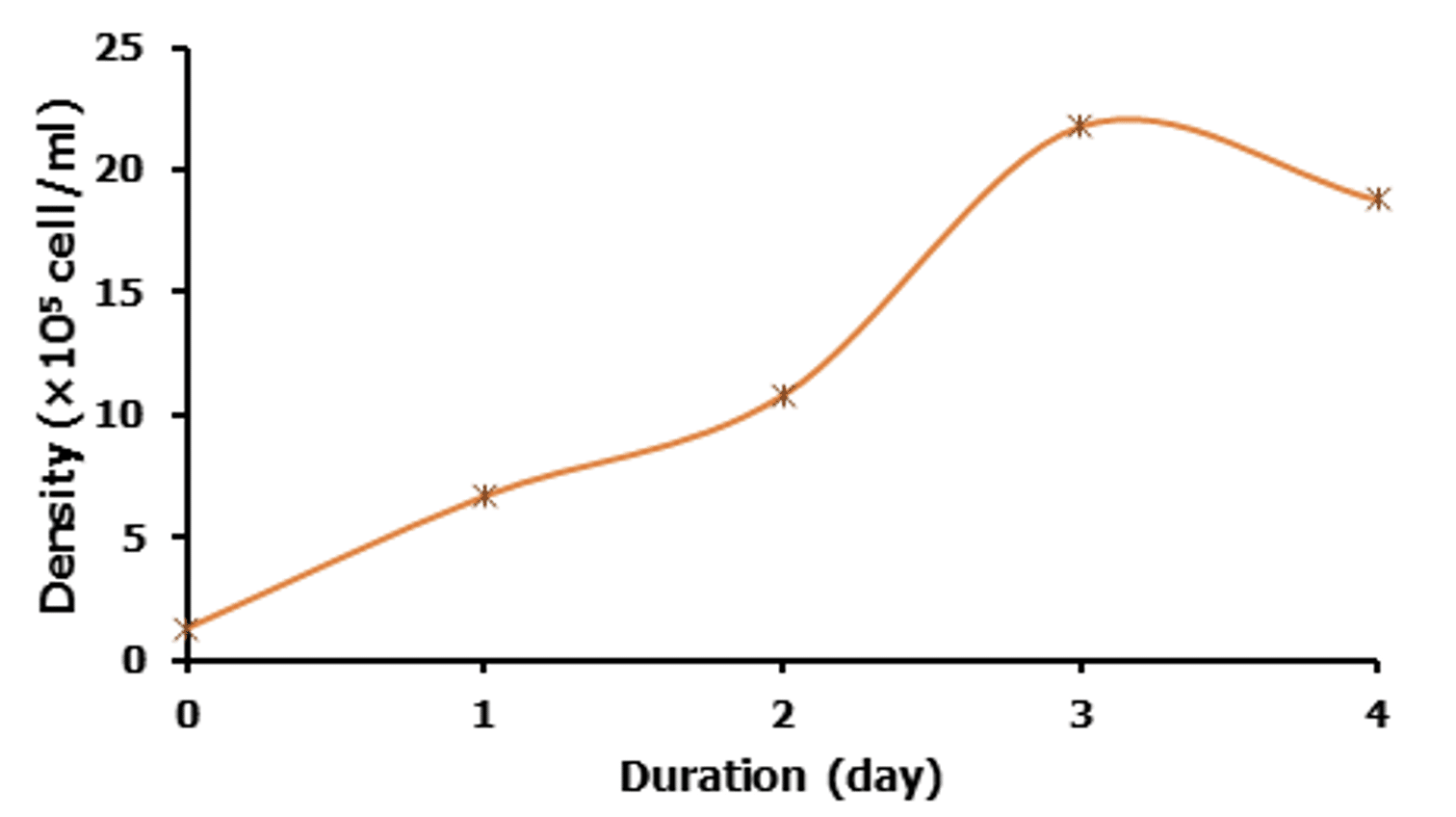
The growth curve analysis showed that C. gracilis responded very well to our modified culture media. The growth curve using 1 ml/L of Urea+TSP, mineral mix+EDTA, Silicate gel, Commercial NPK, Commercial compost showed that exponential growth occurs at day two to three (Figure 3). The initial cell density was 1.3×105 cell/ml that peaked with 21.8×105 cell/ml at day three (Figure 3). Death of algae started after day three and decreased the density to 18.8×105 cell/ml at day four.
3.2 Effect of fertilizer combinations
The effect of different commercial fertilizer combinations in the modified culture media shows that there was significant variation in the cell density of C. gracilis cultured among four combinations over three days in the hatchery facility with local ingredients (Table 4). The significantly higher cell density was observed in T1, where a total combination of urea, TSP, mineral mix, EDTA, silicate gel, NPK, compost were used, than the other treatments where one or more ingredients were missing (Figure 2). The relationship among the treatment shown by the post-hoc tests as T1>T2=T4>T3. The minerals and silicate was common for all the treatments. Addition of only NPK in T3 showed lowest cell density compared to addition of NPK and compost in the T4 (Figure 4). However, T2 and T4 did not differ significantly.
Table 3. The difference in average (±sd) cell density (×105 cell/ml) of Chaetoceros gracilis in different treatments with fertilizers (T1-T4) and vitamins (V0-V2). NB. Superscripts were assigned through one way ANOVA and Tukey’s post-hoc test at 0.05%. Data range in parenthesis.
3.3 Effect of vitamin supplimentation
The addition of vitamin with the best performing fertilizer combinations found from experiment 01 showed that significant increase of the cell density of C. gracilis was achieved with Neobion supplement in V2. However, the addition of multivitamin-Zinc B reduced the cell density in treatment V1 compared to control (V0) where no vitamins were added (Figure 4).
3.4 Continuous mass culture of C. gracilis
In case of mass culture of C. gracilis, it took a total of 21 days to reach a 300 L culture volume from a starter culture volume 200 ml (Table 3). The initial C. gracilis cell density in mass culture trial at 300 L culture volume was 6.6×105 cell/ml which peaked at day two 23.8 ×105 cell/ml due to the inclusion of vitamin Neobion. Though a slight decrease in cell concentration was seen at day four, it was possible to continuously grow in 300 L tank over two weeks with regular supply of media and filtered sea water (Figure 5). However, density was monitored up to day eight.


4. Discussion
The growth curve of marine diatom C. gracilis with the locally available fertilizers combination demonstrated the exponential growth with the promising cell density (21.8×105 cell/ml) occurred from day two to three. Usually the exponential growth of C. gracillis is achieved within 2-3 days interval in batch culture methods (Castro et al., 2022 ![]() ). Perez et al. (2017
). Perez et al. (2017 ![]() ) found that the C. gracilis cell doubling time ranges from 2.79–2.87 days in photobioreactors and outdoor cultivation system. Qayyum et al. (2020
) found that the C. gracilis cell doubling time ranges from 2.79–2.87 days in photobioreactors and outdoor cultivation system. Qayyum et al. (2020 ![]() ) found that fertilizer-based media was comparable with Bold Basal media in terms of growth parameters, cell density and doubling time for Dictyosphaerium. Moreover, comparable cell growth and density was found using fertilizer based media contrast to costly F2 media (Ribeiro et al., 2020
) found that fertilizer-based media was comparable with Bold Basal media in terms of growth parameters, cell density and doubling time for Dictyosphaerium. Moreover, comparable cell growth and density was found using fertilizer based media contrast to costly F2 media (Ribeiro et al., 2020 ![]() ). Present study shows that agricultural fertilizers have the significant impact on the growth of C. gracilis and can be an alternative to pure culture media. Diatoms play a significant role in global carbon fixation and contribute 40% to the oceanic primary productivity that makes them important organisms in the context of sustainable biotechnology (Bhattacharjya et al., 2024
). Present study shows that agricultural fertilizers have the significant impact on the growth of C. gracilis and can be an alternative to pure culture media. Diatoms play a significant role in global carbon fixation and contribute 40% to the oceanic primary productivity that makes them important organisms in the context of sustainable biotechnology (Bhattacharjya et al., 2024 ![]() ). Their ability to efficiently sequester carbon and assimilate bioactive compounds renounces them as a valuable resource for wastewater bio-refineries. Diatoms can be utilized for producing high-demand products such as aqua-feed, biodiesel, and bio-fertilizers (Ahmad et al., 2022
). Their ability to efficiently sequester carbon and assimilate bioactive compounds renounces them as a valuable resource for wastewater bio-refineries. Diatoms can be utilized for producing high-demand products such as aqua-feed, biodiesel, and bio-fertilizers (Ahmad et al., 2022 ![]() ; Mahata et al., 2022
; Mahata et al., 2022 ![]() ). However, the successful cultivation of diatoms at an industrial scale is intricately linked to their growth dynamics, which are highly sensitive to environmental conditions and culture medium composition (Murison et al., 2023
). However, the successful cultivation of diatoms at an industrial scale is intricately linked to their growth dynamics, which are highly sensitive to environmental conditions and culture medium composition (Murison et al., 2023 ![]() ).
).
The combinational effects of commercial fertilizer components (urea, TSP, NPK, compost) with mineral mix, EDTA, silicate gel doubled up the cell growth of C. gracilis than the other treatments that lacks one or more fertilizer components. Agricultural fertilizers, widely used in crop production, contain essential nutrients such as nitrogen, phosphorus, and potassium, which are critical for microalgal growth (López-Ruiz et al., 1995 ![]() ; Tahiri et al., 2024
; Tahiri et al., 2024 ![]() ). To address the cost barriers in large-scale microalgae cultivation, agricultural fertilizers have been identified as a practical and cost-efficient alternative to expensive culture media (Jbari et al., 2020
). To address the cost barriers in large-scale microalgae cultivation, agricultural fertilizers have been identified as a practical and cost-efficient alternative to expensive culture media (Jbari et al., 2020 ![]() ; Qayyum et al., 2020
; Qayyum et al., 2020 ![]() ; Tahiri et al., 2024
; Tahiri et al., 2024 ![]() ; Ribeiro et al., 2020
; Ribeiro et al., 2020 ![]() ). Their use in microalgae cultivation not only reduces input costs but also simplifies the supply chain, as these fertilizers are readily available and do not require the complex manufacturing processes associated with specialized culture media Chakraborty et al. (2023
). Their use in microalgae cultivation not only reduces input costs but also simplifies the supply chain, as these fertilizers are readily available and do not require the complex manufacturing processes associated with specialized culture media Chakraborty et al. (2023 ![]() ). By utilizing the agricultural fertilizers, companies can enhance the economic viability of microalgae-based technologies, making large-scale production more feasible and competitive with traditional energy and product sources (Qayyum et al., 2020
). By utilizing the agricultural fertilizers, companies can enhance the economic viability of microalgae-based technologies, making large-scale production more feasible and competitive with traditional energy and product sources (Qayyum et al., 2020 ![]() ). The widespread availability and affordability of these fertilizers make them particularly suited for industries focusing on biofuel production, bioremediation, and the generation of high-value compounds such as nutraceuticals (Jbari et al., 2020
). The widespread availability and affordability of these fertilizers make them particularly suited for industries focusing on biofuel production, bioremediation, and the generation of high-value compounds such as nutraceuticals (Jbari et al., 2020 ![]() ; Mahata et al., 2022
; Mahata et al., 2022 ![]() ; Tokushima et al., 2016
; Tokushima et al., 2016 ![]() ).
).
Another key factor influencing the cost of microalgae production is the use of vitamins in culture media. C. gracilis exhibited robust growth following the application of Neobion (that contain vitamin B1, B6 and B12) with agriculture fertilizer, highlighting a potential to reduce costs associated with expensive vitamin supplements. While without Neobion cell density peaked at day three, with Neobion supplementation cell growth peaked at day two emphasizing the beneficial role of vitamin addition in the media (Croft et al., 2006 ![]() ). Many species of phytoplankton require vitamin B for optimal growth, and their absence can severely hinder the growth rates (Durham et al., 2015
). Many species of phytoplankton require vitamin B for optimal growth, and their absence can severely hinder the growth rates (Durham et al., 2015 ![]() ; Helliwell, 2017
; Helliwell, 2017 ![]() ). However, some microalgae demonstrate the ability to grow without specialized vitamin supplementation (Helliwell, 2017
). However, some microalgae demonstrate the ability to grow without specialized vitamin supplementation (Helliwell, 2017 ![]() ). This finding is particularly relevant for industries looking to optimize the cost-efficiency of large-scale phytoplankton cultivation by focusing on species like C. gracilis, industries can eliminate the need for costly vitamins, significantly lowering the overall cost of production while maintaining high biomass yields.
). This finding is particularly relevant for industries looking to optimize the cost-efficiency of large-scale phytoplankton cultivation by focusing on species like C. gracilis, industries can eliminate the need for costly vitamins, significantly lowering the overall cost of production while maintaining high biomass yields.
Multivitamin Zinc B addition reduced the cell density in C. gracilis, indicating that there may be inhibitory effect of some trace metals. Zinc can either stimulate or inhibit algae growth, with low concentrations promoting growth and high concentrations slowing it and reducing cell division (El-Agawany and Kaamoush, 2023 ![]() ). Since the concentration of Zinc was not determined, therefore, the exact cause of cell growth reduction in multivitamin Zinc B is not clear.
). Since the concentration of Zinc was not determined, therefore, the exact cause of cell growth reduction in multivitamin Zinc B is not clear.
In addition to agricultural fertilizers and vitamins, minerals have promising option for lowering the cost of industrial-scale microalgae production. Minerals are crucial and required in small amount but have the great impact on the growth of microalgae. Absence of those minerals may inhibit the growth of microalgae. For example, decreased silicate availability reduce the long-chain and unsaturated fatty acids in diatoms (Dineshbabu et al., 2019 ![]() ). By using mineral fertilizers, industries can not only lower their operational costs but also align with sustainable practices, as these fertilizers can often be sourced from existing agricultural supply chains. Nutrient availability and environmental factors can significantly impact the size and growth rate of diatoms, and similar growth variations have been observed in Phaeodactylum tricornutum under different nutrient stress conditions (Bhattacharjya et al., 2024
). By using mineral fertilizers, industries can not only lower their operational costs but also align with sustainable practices, as these fertilizers can often be sourced from existing agricultural supply chains. Nutrient availability and environmental factors can significantly impact the size and growth rate of diatoms, and similar growth variations have been observed in Phaeodactylum tricornutum under different nutrient stress conditions (Bhattacharjya et al., 2024 ![]() ). This variability in growth response underscores the need for precise optimization of cultivation parameters such as amount of trace metals, light, and temperature to enhance yield and productivity in large-scale diatom-based applications (Kholssi et al., 2023
). This variability in growth response underscores the need for precise optimization of cultivation parameters such as amount of trace metals, light, and temperature to enhance yield and productivity in large-scale diatom-based applications (Kholssi et al., 2023 ![]() ).
).
Marine diatom C. gracilis culture within the hatchery facility using a modified fertilizer media developed from locally available fertilizers and vitamin clearly exhibit that mass culture of algae without pure and costly culture media is possible and may play a significant role in the sustainable development of hatchery and aquaculture operations. The next step on the scaling up of mass culture of C. gracillis in the hatchery facility signifies the potential of live feed production in the mollusks, crustacean and fish hatchery without highly decorated laboratory facilities (Perez et al., 2017 ![]() ). This may also enhance the potential zooplankton culture in the hatchery i.e. rotifer culture. At least, this finding increase our hope that in the natural environmental condition without controlled temperature and pure culture set up, algae can be cultured for hatchery operations which may enhance the bivalve hatchery development, culture and brood rearing within indoor facility. Continuous culture of C. gracillis will be worthy as the bacillariophycean species give the best food value achieved for bivalve comparing with other algal classes (Dineshbabu et al., 2019
). This may also enhance the potential zooplankton culture in the hatchery i.e. rotifer culture. At least, this finding increase our hope that in the natural environmental condition without controlled temperature and pure culture set up, algae can be cultured for hatchery operations which may enhance the bivalve hatchery development, culture and brood rearing within indoor facility. Continuous culture of C. gracillis will be worthy as the bacillariophycean species give the best food value achieved for bivalve comparing with other algal classes (Dineshbabu et al., 2019 ![]() ; Ma and Hu, 2024
; Ma and Hu, 2024 ![]() ). However, the methods need further optimization through advanced research, particularly by adopting techniques such as perfusion, photobioreactors, and industrial biotechnology to enhance algae productivity and biomass yield (Tachihana et al., 2023
). However, the methods need further optimization through advanced research, particularly by adopting techniques such as perfusion, photobioreactors, and industrial biotechnology to enhance algae productivity and biomass yield (Tachihana et al., 2023 ![]() ).
).
4. Conclusions
This study demonstrates that locally sourced, affordable fertilizers and vitamins can effectively replace expensive laboratory-grade media for C. gracilis cultivation. Among the fertilizer combinations tested, the T1 recipe (urea, TSP, mineral mix, EDTA, silicate gel, NPK, and compost) yielded the highest cell density. Additionally, Neobion supplementation further enhanced cell growth, while multivitamin-Zinc B proved less effective. The continuous culture trial using Neobion in a larger tank maintained a high cell density, suggesting the viability of this modified media for large-scale microalgae cultivation in aquaculture hatcheries. Microbial contamination was seen in the continuous culture system which needs to be addressed in future research how to prevent contamination in a cost-effective and handy manner. Optimizing growth conditions, utilization of bioactive compounds and industrial use of microalgae will be supporting sustainability and promoting renewable resources for energy, environmental remediation.
Acknowledgements
Authors acknowledge the facilities provided by the Director and hatchery personnel of the Coastal Biodiversity, Marine Fisheries and Wildlife research Institute, CVASU within the hatchery. Authors are grateful to Dr. Helena Khatun and Mahima Ranjan Acharjee, Department of Aquaculture, CVASU for supplying algal stock.
Data availability
The data generated from this study might be shared with a valid request from the corresponding author.
Funding details
This work was supported by Sustainable Coastal and Marine Fisheries Project (SCMFP ID L2W1-03) funded by the Department of Fisheries (DoF), Bangladesh and World Bank granted to MSR Khan.
Informed consent statement
Not required.
Conflict of interest
The authors report there are no competing interests to declare.
Author contributions
PB: conceptualization, methodology, formal analysis, investigation, data curation, writing; MMK: writing—review and editing; MSR Khan: conceptualization, methodology, Validation, supervision, funding, writing—review and editing. All the authors critically reviewed the manuscript and agreed to submit a final version of the article.