Climate change poses a serious threat to agriculture, considerably impeding agricultural output by altering weather patterns and escalating extreme occurrences like heat waves, severe droughts, salinity, and floods, which are made worse by biotic stressors such as pathogens, nematodes, insects, and weeds (Delarue et al., 2025). Drought, salinity, and heat stress are critical abiotic factors that adversely impact plant growth, development, and reproductive processes (Sato et al., 2024). This poses a serious threat to food security and causes substantial losses in agricultural products. To address this pressing challenge, plant breeding plays an important role in developing climate-resilient crops, thereby ensuring sustainable food production. Around 10,000 years ago, ever since the domestication of plants began, plant breeding has been extensively successful in developing crops and varieties that are essential to the development of modern society, and consistently outperformed (neo-)Malthusian predictions (Fedoroff, 2010). Conventional breeding methodologies have played a pivotal role in developing modern cultivars, substantially enhancing the yield of key crops since the middle of the 20th century onward (Perez-de-Castro et al., 2012). Over the past 150 years, plant breeding has had significant success in reducing the life cycle of crop plants, enabling other major crops o integrate in one cropping year (Ahmar et al., 2020). It is projected that the world population will be 9.7 billion by 2050, and by 2100, there will be roughly 10.9 billion (Gu et al., 2021). The global need for food will significantly increase substantially to address the demands of an expanding population, whereas several biotic and abiotic challenges imposed by climate change continue to impede crop productivity (Gull et al., 2019). In order to satisfy the demand of the expanding population, food production must increase by an estimated 70-100% (Foley et al., 2011). Conventional breeding is a time-consuming process, often requiring over 10 years to develop and release a new cultivar (Bharti and Chimata, 2019). Dealing with complex traits like abiotic and biotic stress tolerance/resistance is complicated and time consuming in conventional breeding and are significantly influenced by genotype-environment interactions leading to inaccuracies in breeding objectives (Lema, 2018). On the contrary, genomic approaches are advantageous for investigating these complex traits, as such characteristics are typically polygenic and significantly affected by environmental influences. In this modern era, breeders can use genomics-based methods and sophisticated molecular tools, including ‘genomic selection’, ‘markers-assisted selection’ (MAS), ‘marker-assisted backcrossing’ (MABC), ‘quantitative trait loci’ (QTL) mapping, and ‘genome editing’ (Figure 1), to facilitate precise and efficient genetic improvements (Kumar et al., 2024).
Genomic election has effectively identified small-effect genes associated with multiple QTLs, which are important in plant breeding as they regulate the majority of important economic and agronomic traits (Robertsen et al., 2019). MAS is a technique that employs DNA markers to recognize the genomic regions to a specific traits in plants (Das et al., 2017).
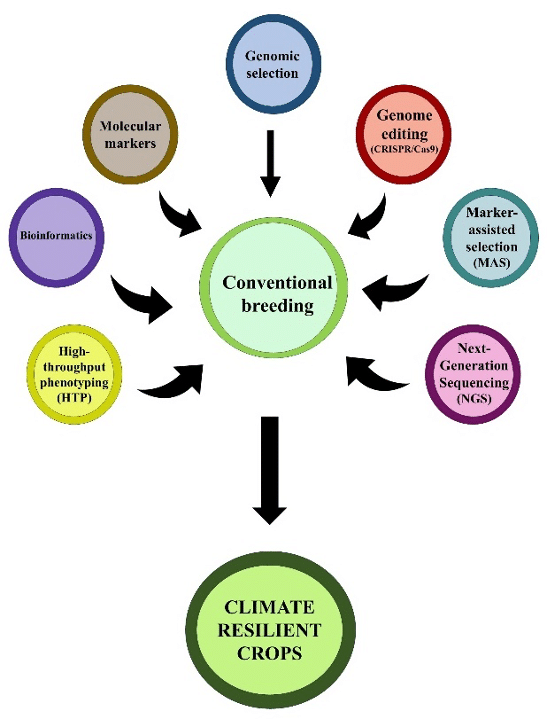
Molecular markers (SNP, InDel, RAPD, SSR, ISSR, etc.) play a vital role in QTL mapping of stress-related genes, such as drought and salinity tolerance genes, which regulate responses to these environmental stresses (Younis et al., 2020). High-throughput phenotyping (HTP) enables detailed information on diverse phenotypic traits like stress tolerance, disease resistance, growth, and crop yield (Shakoor et al., 2017). A cutting-edge genome editing technique, CRISPR-Cas9 holds great promise for use in contemporary crop breeding (Riaz et al., 2021; Dutta, 2024). It has shown substantial potential in the development of climate-resilient crop cultivars (Razzaq et al., 2022). With the advancement in science and technologies, plant breeding is also evolving day by day. The prospect of plant breeding is dependent on technological advances through the application of molecular tools, genomic technologies, precision breeding technologies, and the development of crop varieties resilient to stresses, contributing to worldwide food security. The advanced genomics tools not only save the time of the breeding program but are also of great value for the transfer of complex traits to tackle the issues of food security, climate change impacts, and sustainable crop production. Improving the productivity, efficiency, and speed of conventional breeding programs, requires the integration of molecular tools to develop climate-resilient crop varieties.
Acknowledgements
None to declare.
Ethical approval statement
None to declare.
Data availability
Not applicable.
Informed consent statement
Not applicable.
Conflict of interest
The authors declare no conflict of interests.
Author contributions
Conceptualization: Md. Abdur Rahim; Writing original drat: Md. Nahid Hasan and Md. Abdur Rahim; Figure preparation: Md. Nahid Hasan; Final reviewed and revised: Md. Abdur Rahim. Both authors critically reviewed and edited the manuscript and agreed to submit the final version of the editorial.



