Fisheries and aquaculture sectors are integral in achieving the sustainable development goals (SDGs) set out by the 2030 Agenda through the Food and Agriculture Organization of the United Nation. Global fish production had reached approximately 223.2 million tonnes, valued approximately USD 452 billion, of this, aquaculture contributed 94.4 million tonnes, valued at USD 296 billion (FAO, 2024). It is worth to note that for the first time, aquaculture production of aquatic animals surpassed capture fisheries, contributing 94.4 million tonnes, which is 51% of the total aquatic animal production. This growth has been achieved due to modern aquaculture practices, advanced technological innovations, and enhanced management strategies that have optimized production efficiency and sustainability to meet global demand (Ahmad et al., 2020; Freitas et al., 2020). However, escalating production costs, suboptimal yields, environmental discharge of effluents, and increasing water scarcity have driven scientists to explore alternative approaches (Allen and Steeby, 2011). Aquaculture systems like biofloc, raceway, in-pond raceway, and integrated multi-trophic aquaculture have been introduced globally to boost production efficiency, improve water quality, and enhance sustainability (Lal et al., 2024). Biofloc technology (BFT) is a modern aquaculture method that utilizes microbial communities to manage water quality and enhance productivity (Yu et al., 2023). The origins of BFT date back to the early 1970s at the French Research Institute for Sea Exploration, Pacific Oceanic Center (El-Sayed, 2021). BFT offers opportunities to boost fish biomass, enhance biosecurity, improve feed conversion ratios (FCR), optimize aquaculture efficiency, increase feed recyclability, and minimize the need for water exchange (Huang et al., 2022). Biofloc technology is being increasingly adopted in commercial fish and shellfish farms across countries like India, Indonesia, Thailand, Malaysia, South Korea, and China, with applications ranging from small to large-scale operations (Kuhn et al., 2015). This global trend has reached Sylhet region of Bangladesh where farmers are exploring biofloc as a sustainable alternative to traditional aquaculture methods amidst declining natural resources. Since its introduction, BFT has gained popularity across various parts of Bangladesh and can even be set up in small backyard spaces. It is particularly appealing to unemployed youths, offering the potential for higher profits with lower production costs and minimal space requirements. Fish farmers in biofloc system often complained about the outbreak of infectious and non-infectious diseases. Disease outbreaks are often associated with poor water quality condition of biofloc farms. Nevertheless, BFT encounters several challenges, such as elevated nitrogen levels and a higher incidence of disease (Boyd et al., 2020; Kunwong et al., 2022). The zero water exchange in the biofloc farms lead to poor water condition. The water quality parameters like dissolved oxygen, total organic load, ammonia and nitrites are major factors that influence both parasitic and bacterial infections. Several studies (Walakira et al., 2014; Yilmaz et al., 2023) demonstrated a positive correlation of disease incidences with water quality alterations. Although knowledge regarding disease prevalence could help farmers avoid and control infections in aquaculture farms, almost no survey has been conducted to determine the status of diseases in biofloc farms in the north-eastern region of Bangladesh. It is very important to know the prevalent diseases, operational challenges, and long term sustainability issues of biofloc aquaculture systems in the Sylhet region of Bangladesh. We hypnotize that biofloc aquaculture systems faces multiple challenges with disease outbreak, frequent power outages, trained biofloc aquaculturists and suboptimal water quality management may impede its sustainability. So, we aimed to report the potentiality of BFT by knowing the disease prevalence, stocking density, major problems and challenges and the long-term viability of this culture system in the Sylhet region based on survey work. Survey data alone is occasionally inadequate for identifying the specific cause of disease occurrence. In that case, the present study was further carried out through laboratory diagnosis. Channa striata is a popular fish in developing countries like Bangladesh for its boneless tasty flesh, a high price in the market, alluring flavor, and some medicinal value (Sinh et al., 2014; Sahid et al., 2018; Wahab et al., 2015). Carp, catfish, tilapia, and shrimp are commonly cultivated in most biofloc systems, and more recently, morrels (Channa spp.) have also been introduced into biofloc aquaculture (Alam and Khan, 2024; Raza et al., 2024). While murrel fish are considered disease-resistant, they are still susceptible to infections like tail rot, hemorrhagic septicemia, and leech infestations (Kolupula et al., 2022). To the best of our knowledge, there have been no reports of disease outbreaks in C. striata within biofloc aquaculture systems in Bangladesh. To address this gap, diseased C. striata specimens were collected, and bacteriological studies, biochemical tests, microscopic analyses, and histopathological examinations were conducted to better understand the infections. Identifying the type of infection is crucial for effective disease prevention and treatment. The results of the present study will provide valuable information for managing fish diseases and treatments, as well as understanding the current status of biofloc technology.
2. Materials and Methods
2.1 Ethical approval
The Animal Ethics Committee of Sylhet Agricultural University provided guidelines for the handling of fish in this study (Memo: SAU/AEC/FOF/FHM-550), which were strictly followed.
2.2 Study area
The study was conducted on nine biofloc fish farms distributed across three Upazilas, Sylhet Sadar, Gowainghat, and Dakshin Surma in Sylhet district, situated in the northeastern region of Bangladesh (Figure 1).
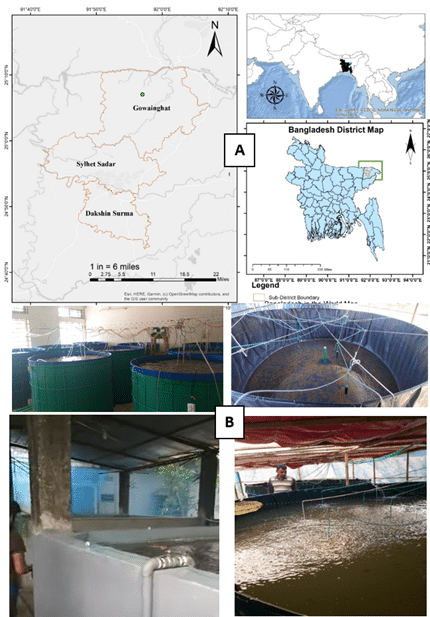 Figure 1 (A). Map of Bangladesh showing the upazilas of Sylhet district. The survey was conducted in three upazilas namely, Sylhet Sadar, Gowainghat, and Dakshin Surma, which are highlighted, disease fish were collected from Gowaingaht upzila (green circle) (B). Different patterns of biofloc culture setups observed during the survey.
Figure 1 (A). Map of Bangladesh showing the upazilas of Sylhet district. The survey was conducted in three upazilas namely, Sylhet Sadar, Gowainghat, and Dakshin Surma, which are highlighted, disease fish were collected from Gowaingaht upzila (green circle) (B). Different patterns of biofloc culture setups observed during the survey.2.3 Data collection methods
Data collection was conducted using a combination of structured questionnaire-based interviews and personal interactions with farm proprietors and cultivators. The questionnaire was meticulously designed to align with the objectives of the study, incorporating both closed and open-ended questions to capture quantitative and qualitative data. This approach ensured a comprehensive understanding of the challenges, practices, and perceptions of biofloc technology users.
2.4 Fish samples and clinical investigation
Diseased fish were collected from Ayesha Poultry and Fishery Bohor, Gowainghat (Latitude: 25°06’10.08” N; Longitude: 91°53’30.12” E). The biofloc system consisted of a cemented pond of 10 decimals (length 35 m breadth 12 m). On 21st November 2021, a sudden disease outbreak in C. striata resulted in acute to sub-acute mortality, affecting up to 80% of the stock. The affected fish weighed between 20 g and 200 g. Water quality measurements were taken using a digital multi-parameter probe (HI 9828, YSI Incorporation, Yellow Springs, OH, USA). Postmortem examinations were conducted on 50 moribund or recently deceased C. striata within 24 hours of death at the Laboratory of Fish Disease Diagnosis and Pharmacology, Sylhet Agricultural University. The analyses included parasitological, bacteriological, and histopathological assessments.
2.5 Observation of parasite
Parasites were observed by preparing wet mounts according to the method described by Noga (2014). Small samples of skin from infected fish and mucus from the body surface were carefully collected and placed on glass slides with physiological saline. These samples were then examined under a light microscope to identify ectoparasites. Observations and identifications were conducted using an Olympus CX41 microscope (Japan), and the findings were documented photographically (Mamun et al., 2021a). The investigations were carried out at the Laboratory of Fish Disease Diagnosis and Pharmacology, Sylhet Agricultural University, Bangladesh.
2.6 Biochemical analysis of isolated bacteria
Biochemical tests, including Gram staining, catalase test, motility test, MR-VP (Methyl Red and Voges–Proskauer) test, indole test, citrate utilization, and carbohydrate (CHO) fermentation test, were conducted to characterize and identify the bacterial isolates. These analyses were performed following standard microbiological protocols at the Microbiology and Immunology Laboratory, Faculty of Veterinary, Animal, and Biomedical Sciences, Sylhet Agricultural University.
2.7 Antibiotic sensitivity test
The antibiotic sensitivity of the isolated bacteria was evaluated using the disc diffusion method. Bacterial broth cultures were uniformly spread over Mueller-Hinton Agar (MHA) plates and incubated at 37 °C for 24 hours. The assay, performed for 15 antimicrobials (HiMedia, India), according to the standard procedures outlined by the Clinical and Laboratory Standards Institute (CLSI, 2020).
2.8 Histopathological investigation
For histological examination, tissues, including skin, muscle, gill, liver, spleen, and kidney, were carefully collected after euthanizing the fish with an overdose of MS-222 (180 mg/L). The samples were promptly fixed in 10% neutral buffered formalin for 48–72 hours to preserve cellular structure and prevent autolysis. Following fixation, the tissues were processed through standard histological procedures, including dehydration in graded ethanol concentrations, clearing with xylene, and embedding in paraffin wax. Thin sections, 4–6 µm in thickness, were prepared using a rotary microtome and mounted on glass slides. These sections were stained with hematoxylin and eosin (H&E) to observe cellular and tissue morphology. Stained slides were examined under a light microscope (Carl Zeiss Primostar 3, Germany), and images were captured using a digital imaging system for a detailed pathological analysis.
2.9 Isolation and molecular identification of bacterial strain
According to a set methodology, A. veronii were isolated from diseased fish (C. striata) (Mamun et al., 2020). The isolation process utilized Rimler-Shotts (RS) selective medium (Himedia, Mumbai). The external surface of the diseased fish was sanitized using 70% ethanol. The renal and gill surfaces of the diseased fish were swabbed, and the obtained samples were streaked onto RS agar plates. On the RS medium, yellow colonies were produced following a day of incubation at 37℃. Pure yellow colony streaks from RS agar plates were randomly chosen and positioned on Brain Heart Infusion (BHI) agar (Himedia, Mumbai) slants. The DNA of Aeromonas veronii was isolated from a pure culture using the Maxwell Blood DNA Kit (Model: AS1010, Promega, USA). PCR amplification of the 16S rRNA gene was carried out using the Hot Start Green Master Mix from Promega (USA), which contains dNTPs, buffer, MgCl₂, and Taq Polymerase. Universal primers 27-F (5′-AGA GTT TGA TCM TGG CTC AG-3′) and 1492-R (5′-CGG TTA CCT TGT TAC GAC TT-3′) were used. The PCR protocol followed the methods described in our previous study (Rahman et al., 2021). Following PCR amplification, the products were purified and subjected to sequencing PCR using the Big Dye Terminator kit with either the forward or reverse primer. The DNA template was then precipitated with ethanol and sequenced using a 3500xL Genetic Analyzer (Applied Biosystems).
2.10 Submission of bacterial sequence in the NCBI (National Centre for Biotechnology Information)
The raw sequence data were assembled and checked for quality using BioEdit version 7.0. The assembled sequences were submitted to the GenBank database within the National Centre for Biotechnology Information (NCBI), where unique accession numbers were assigned to each sequence. The edited sequences were aligned against their corresponding genes in the GenBank database using a BLASTN search. Bacterial isolates were identified to the species level when they exhibited ≥99% 16S rRNA sequence similarity to relevant entries in GenBank. Phylogenetic trees were constructed using the maximum likelihood method in MEGA X software with 1000 bootstrap replicates. The analysis utilized default parameters, including a 95% cutoff for partial deletion, uniform rate variation among sites, and consideration of both transversions and transitions. Final groupings into phylogenetic branches were determined by integrating results with the closest matching sequences from GenBank, providing a comprehensive understanding of the bacterial strains.
2.11 Data processing and analysis
The collected data was systematically categorized and Microsoft Excel was employed to process the data, utilizing its features for sorting, filtering, and performing calculations to uncover patterns and trends. Primary data was then cross-referenced with existing literature and previous studies to validate findings and provide contextual relevance. Sequence data was analyzed using Basic Local Alignment Search Tool (BLAST) of National Center for Biotechnology Information (NCBI), USA.
3. Results
3.1 Types of disease observed in survey reports
The survey conducted across nine biofloc fish farms in different Upazilas of Sylhet district revealed the presence of seven distinct diseases with varying prevalence and severity (Table 1). Among these, tail rot was the most prevalent, affecting 55.56% of the farms (Table 2). Characterized by lesions on the tail margin, erosion, and complete loss of the tail, rail rot was primarily managed using antibiotics such as oxytetracycline, ciprofloxacin, and tetracycline, along with herbal aqua drugs like garlic and turmeric. Hypoxia, observed in 44.44% of the farms (Table 2), was identified by gasping at the water surface and open-mouth symptoms in dead fish. This condition was mitigated through the use of commercial oxygen supplements and transferring fish from biofloc systems to ponds. Similarly, dropsy, with a prevalence of 44.44%, was marked by distended abdomens, intestinal inflammation, and protruding scales. Farmers managed dropsy by halting feeding and applying renamycin. Fungal attacks, also prevalent in 44.44% of farms, were associated with body ulceration and treated with herbal remedies, such as garlic and turmeric, supplemented with lime and salt applications. Exophthalmia, reported in 33.33% of the farms (Table 2), was characterized by abnormal protrusion of the eyeball, though no specific control measures were identified. Less common diseases included streptococcosis (11.11%), marked by loss of appetite, skin hemorrhages, and bloody spots inside the opercula, which was treated using ciprofloxacin, tetracycline, gentamicin, and other commercial antibiotics. Similarly, gill clogging, also seen in 11.11% of farms, was linked to reduced respiratory function. Control measures involved reducing feeding frequency and performing water exchanges to improve water quality. The findings of the present study showed a significant disease burden in biofloc aquaculture systems, with multiple diseases frequently co-occurring. Although antibiotics and herbal remedies were commonly employed as control measures, certain conditions, such as exophthalmia, lacked specific management strategies. The data emphasize the need for improved disease management protocols and water quality maintenance to ensure the sustainability of biofloc aquaculture in the Sylhet region.
Table 1. Names, locations, and disease outbreaks in biofloc farms of Sylhet district.
Table 2. Diseases, identifying keys, affected farms, and control measures in biofloc farms of Sylhet district.
3.2 Problems, threats, management, and farmers’ concerns about biofloc fish farms in Sylhet district
Biofloc fish farming in Sylhet district has confronted with various challenges, as summarized in Table 3. The most commonly reported issues included seed shortages and electricity disruptions, both cited by 100% of the respondents. High ammonia levels (66.67%) were another significant concern, followed by high mortality rates (55.56%), oxygen depletion (44.44%), poor growth rates (44.44%), and low-quality probiotics (33.33%). Poor flocculation and untrained culturists were also reported by 33.33% of respondents. Farmers are taken several management strategies to address these issues. High ammonia levels were managed through water changes, the use of iron-free water from reserve tanks, and temporarily feed off. Oxygen depletion was mitigated by increasing aeration, adding more air pumps, using oxygen supplements, and in some cases, transferring fish to ponds. For electricity disruptions, farmers relied on backup power systems, including instant power supply (IPS) units and generators. Despite these efforts, many challenges, such as low-quality probiotics, poor growth performance, and untrained culturists, lacked effective management solutions and often without viable strategies to mitigate their problems.
As shown in Table 4 and Figure 2, seven out of nine farmers (77.78%) expressed that biofloc farming systems were non-sustainable in their current state.
 Figure 2. Perception of sustainability among biofloc farms in Sylhet district, Bangladesh, showing 77.78% considered non-sustainable and 22.22% sustainable.
Figure 2. Perception of sustainability among biofloc farms in Sylhet district, Bangladesh, showing 77.78% considered non-sustainable and 22.22% sustainable.
The reasons cited included frequent disease outbreaks, high mortality rates, poor growth performance, and recurring technical issues such as electricity disruptions and seed shortages. Other concerns included high maintenance costs, untrained personnel, and challenges in controlling water quality parameters like ammonia and dissolved oxygen. Conversely, 22.22% of the farmers viewed biofloc systems as sustainable, primarily attributing their success to high-value fish species, effective flocculation management, and long-term patience with harvest cycles. These farmers highlighted that with proper training, better seed availability, and improved infrastructure, biofloc systems could become a viable and sustainable option for aquaculture in the Sylhet region.
Table 3. Biofloc farms problems/ threats and management in Sylhet district.
Table 4. Farmers’ opinion about biofloc system in Sylhet district.
3.3 Disease diagnosis
3.3.1 Water quality parameters
During sampling, the water exhibited a foul odor, with key parameters recorded as follows: temperature 32±2.0°C, pH 8.8±1.34, dissolved oxygen (DO) 3.5±1.5 ppm, ammonia (NH₃) 2.5±0.51 ppm, total dissolved solids (TDS) 200±40 ppm, salinity 0.09±0.02 ppt, water pressure 755.60±0.60 mm Hg, and conductivity 153.75±8.84 Siemens/m.
3.3.2 Clinical signs
Infested C. striata exhibited anorexia, lethargy, respiratory distress, and abnormal behaviors such as segregated from the school and rubbed their skin against the tank walls or substrate. Moribund fishes showed ulcerative lesions on the skin, discoloration, scale loss, and excessive mucus secretion, with some areas showing sloughed off epidermis (Figure 3A). Severe lesions exposed underlying tissues, while the gills appeared pale and anemic (Figure 3B). Microscopic examination confirmed the presence of trematodes cercariae firmly attached in the skin and muscle of the fish. This cercariae could not identified to genus or species level because they are too young and small, hence they are enlisted here as unidentified.
 Figure 3. A) The dead and infested C. striata specimens with external signs, including ulcerative lesions (arrows, discoloration of the skin, and apparent scale loss B) The opened operculum reveals pale and anemic gill structures (arrow), excessive mucus secretion, and areas where the mucus and epidermis have sloughed off (circle) C) Microscopic view of the trematode cercariae, D) and E) Showing its elongated body and segmented morphology of the cercariae infections.
Figure 3. A) The dead and infested C. striata specimens with external signs, including ulcerative lesions (arrows, discoloration of the skin, and apparent scale loss B) The opened operculum reveals pale and anemic gill structures (arrow), excessive mucus secretion, and areas where the mucus and epidermis have sloughed off (circle) C) Microscopic view of the trematode cercariae, D) and E) Showing its elongated body and segmented morphology of the cercariae infections.3.3.3 Parasitological investigations
The prevalence and intensity of cercariae infection in C. striata were analyzed based on fish weight categories (Table 5). In fish weighing ≤ 50 g, 75% of the hosts (15 out of 20) were positive for cercariae infection, with a mean intensity of 12.15 parasites per host (range 4–23). Among fish weighing ≥ 50 g, 83% of the hosts (25 out of 30) were infected, showing a higher mean intensity of 17.69 parasites per host (range 7–39). These results indicate a higher prevalence and intensity of cercariae infection in larger fish.
Table 5. Prevalence and intensity of cercariae infection in C. striata based on fish weight.
3.3.4 Colony structures, gram stain and biochemical tests
The bacterial isolates were identified as Gram-negative, motile, rod-shaped organisms based on their morphological and biochemical characteristics. Biochemical tests (Table 6) revealed positive results for indole, Voges-Proskauer, oxidase activity, and the fermentation of glucose, sucrose, and mannitol, while negative results were observed for MR, citrate utilization, catalase, and lactose fermentation. Colony morphology on Rimler-Shotts agar (Figure 4B) showed distinct green and yellowish colonies, and Gram staining (Figure 4A) revealed pink-colored rods under the microscope, confirming their Gram-negative nature. These findings indicate that the isolates belong to the genus Aeromonas.
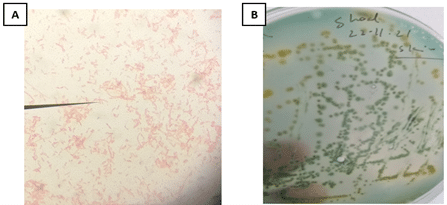 Figure 4. A) Gram staining revealed pink-colored rod-shaped bacteria under the microscope; B) Cultivation on Rimler-Shotts agar demonstrated the formation of distinct green and yellowish colonies, characteristic of Aeromonas spp.
Figure 4. A) Gram staining revealed pink-colored rod-shaped bacteria under the microscope; B) Cultivation on Rimler-Shotts agar demonstrated the formation of distinct green and yellowish colonies, characteristic of Aeromonas spp. Table 6. Colony, morphological and biochemical characteristics of A. veronii
3.3.5 Molecular confirmation of the isolates
PCR amplification of bacterial DNA using 16S rRNA primers (27F and 1492R) successfully produced amplicons of approximately 1500 bp for the bacterial isolates, as shown in Figure 5. Lane M represents the 1 kb DNA ladder, and Lanes 1 to 3 correspond to the bacterial isolates identified in this study. Sequence analysis confirmed the isolates as A. veronii. The phylogenetic tree constructed based on the 16S rRNA gene sequence revealed that the A. veronii isolate from the present study clustered closely with other A. veronii sequences, including ON854128.1, OL873257.1, OR461523.1, OR461524.1, and OR431852.1, with a bootstrap value of 1000 (Figure 6). The isolate was distinct from other bacterial species such as Klebsiella pneumoniae (LC834133.1, LC848124.1) and Lactococcus garvieae (HE962116.1, OX347899.1), which formed separate clusters. This analysis highlights the genetic relationship of the present study's isolate with previously reported A. veronii sequences.
3.3.6 Antibiotic sensitivity test result
Antibiotic susceptibility of A. veronii tested fifteen commercial antibiotics are presented in Table 7. Among the antibiotics, gentamicin (GEN) demonstrated the highest efficacy, producing the largest inhibition zone of 11 mm. Levofloxacin (LE) and novobiocin (NV) also showed strong activity with inhibition zones of 9 mm and 8 mm, respectively. Moderate activity was observed for ciprofloxacin (CIP), erythromycin (E), and streptomycin (S), each with a 7 mm inhibition zone, while co-trimoxazole (COT), azithromycin (AT), and doxycycline (DO) exhibited inhibition zones of 6 mm. Antibiotics such as oxytetracycline (O) and amoxicillin (AMX) displayed weaker activity with inhibition zones of 5 mm and 3 mm, respectively. In contrast, oxacillin (OX), ampicillin (A/S), and ceftriaxone (CTR) showed no or minimal activity, with inhibition zones of 0 mm or 1 mm.
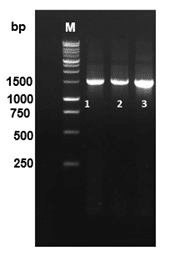 Figure 5. PCR amplification of bacterial DNA using 16s rRNA profile of 27 F and 1492 R primers. Lane M: 1 kb DNA ladder. Lane 1-3: Bacterial isolates (A. veronii).
Figure 5. PCR amplification of bacterial DNA using 16s rRNA profile of 27 F and 1492 R primers. Lane M: 1 kb DNA ladder. Lane 1-3: Bacterial isolates (A. veronii). 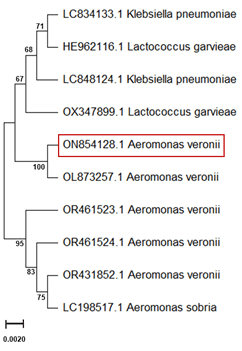 Figure 6. A phylogenetic tree, constructed based on the 16S rRNA gene sequence, illustrates the relationships of A. veronii (marked in red) with closely related species. The tree was created using the Maximum Likelihood approach, with bootstrap values derived from 1000 replicates.
Figure 6. A phylogenetic tree, constructed based on the 16S rRNA gene sequence, illustrates the relationships of A. veronii (marked in red) with closely related species. The tree was created using the Maximum Likelihood approach, with bootstrap values derived from 1000 replicates.Table 7. Antimicrobial resistance and susceptibility of A. veronii.
3.3.7 Histopathological changes in different organs of diseased C. striata
Histopathological examination of diseased C. striata revealed extensive abnormalities across multiple organs. The gills showed loss of secondary gill lamellae. The skin exhibited erosion of the epidermal layer, inflammation, and congestion. The liver displayed vacuole formation, hepatocyte necrosis, and hematolysis. The kidney showed necrosis of hematopoietic tissue, necrotic debris in renal tubules, sloughing of epithelial cells, disrupted glomerulus, melano macrophage centers, disrupted kidney tubules, and loss of kidney tubules. These findings demonstrate systemic damage with inflammation, congestion, and necrosis affecting vital organs in C. striata.
 Figure 7. Histopathological changes of various organs in diseased C. striata. (A) Hypertrophied primary gill lamellae (HPG), gill showing loss of secondary gill lamellae (LSGL); inflammatory cell infiltration; (B) Skin and muscle tissue showed muscle fiber degeneration (MFD), necrotic muscle fibers (NMF), infiltration of erythrocytes (IER), structural damage (SD) and inflammatory responses (IR); (C) Liver tissue revealed vacuolar degeneration or steatosis (VD), cellular disruption and necrosis (CDN), infiltration of inflammatory cells (IIC); (D) Kidney histopathology revealed loss of epithelial integrity (LEI) and sloughed off cells (SoC) into the tubular lumen (EISC), proteinaceous material (PM) Vascular Congestion (VC), Tubular Degeneration (TD) and infiltration of inflammatory cells (IIC), melano-macrophage centre (MMC).
Figure 7. Histopathological changes of various organs in diseased C. striata. (A) Hypertrophied primary gill lamellae (HPG), gill showing loss of secondary gill lamellae (LSGL); inflammatory cell infiltration; (B) Skin and muscle tissue showed muscle fiber degeneration (MFD), necrotic muscle fibers (NMF), infiltration of erythrocytes (IER), structural damage (SD) and inflammatory responses (IR); (C) Liver tissue revealed vacuolar degeneration or steatosis (VD), cellular disruption and necrosis (CDN), infiltration of inflammatory cells (IIC); (D) Kidney histopathology revealed loss of epithelial integrity (LEI) and sloughed off cells (SoC) into the tubular lumen (EISC), proteinaceous material (PM) Vascular Congestion (VC), Tubular Degeneration (TD) and infiltration of inflammatory cells (IIC), melano-macrophage centre (MMC). 4. Discussion
In the aquaculture system of Bangladesh, a wide range of diseases are prevalent, including those caused by bacterial, viral, and fungal pathogens, as well as parasitic infections such as protozoans, monogeneans, digeneans, anchor worms and copepods (Ehsan et al., 2023; Hamom et al., 2020; Debnath et al., 2020; Mamun et al., 2016; Khalil et al., 2014). While numerous reports have documented disease outbreaks in traditional aquaculture systems in Bangladesh, studies on disease prevalence in biofloc farming systems, remain significantly limited (Mawa et al., 2024). This is not unexpected, as biofloc is a relatively recent and modern aquaculture system that has only recently gained widespread adoption globally, including in Bangladesh (Shamsuddin et al., 2022). To the best of our knowledge, this is the first study to document a co-infection involving bacterial and parasitic pathogens in Bangladesh within a biofloc system. The current study in biofloc farms of Sylhet district identified a total of seven diseases or clinical signs, with prevalence rates ranging from 11.11% to 55.56%. Tail rot was the most common condition, affecting 55.56% of the farms, followed by hypoxia, dropsy, and fungal infections, each with a prevalence of 44.44%. Exophthalmia was observed in 33.33% of farms, while bacterial diseases, including streptococcosis, and gill clogging were less frequent, each with a prevalence of 11.11%. Previous studies conducted by Hasan et al. (2013) highlighted the prevalence of various diseases such as pop eye (57.78%), ventral reddening (55.55%), tail and fin rot (48.89%), haemorrhagic lesions over the body surface (45.56%), dropsy (40%), gill rot (40%), white spot (40%), and epizootic ulcerative syndrome (EUS) (33.33%) in pond aquaculture across Mymensingh, Pabna, and Bogra districts in Bangladesh. According to Deb (2018) the most common diseases in different aquaculture farms in Sylhet districts were EUS (27.77%), Argulosis (16.67%), fin and tail rot (19.44%), dropsy (25%), and hypoxia (11.11%). Rahman et al. (2018) found that environmental problems (100%) are the major issue followed by argulosis (68%), dropsy (68%), fin and tail-rot (45%), streptococcosis (28%), EUS (23%) and nutritional diseases in Sylhet division. Recently Mawa et al. (2024) reported a mass mortality event of Gangetic Mystus (Mystus cavasius) in a biofloc farming system, attributed to an acute infection caused by A. veronii, is also evident in our study. Tilapia raised in Indonesian biofloc systems face the threat of protozoan ectoparasites like Glossatella sp., Trichodina sp., as well as Platyhelminthes Gyrodactylus sp., and Dactyrogylus sp., as outlined in Iswari et al. (2020). Meanwhile, Salsabilla et al. (2021) identified three distinct ectoparasites- Trichodina sp., Dactylogyrus sp., and Vorticella sp. - within biofloc farms. The current study highlights significant threats and challenges in biofloc farms in the Sylhet district, directly impacting their sustainability. High ammonia levels (66.67%), oxygen depletion (44.44%), and high mortality rates (55.56%) were identified as critical obstacles. Additional challenges, such as poor flocculation (33.33%), low-quality probiotics (33.33%), and untrained culturists (33.33%), further impede the implementation of sustainable practices. Moreover, the unavailability of quality seeds and electricity disruptions, reported by 100% of respondents, emerged as major barriers to success. Notably, 77.78% of farmers considered the biofloc system non-sustainable, largely due to these unresolved challenges. Similarly, Shamsuddin et al. (2022) reported that the unavailability of skilled workers, scarcity of quality seeds, and frequent power outages pose significant threats. Despite these challenges, biofloc farming offers significant opportunities for income generation and employment, with sustainability requiring targeted research on aerator optimization, system integration, beneficial microorganisms, and floc monitoring, alongside practical interventions such as certified hatcheries, structured training, affordable loans, and reliable, low-cost electricity to enhance its long-term viability (Bossier and Ekasari, 2017; Shamsuddin et al., 2022). In the current study, higher ammonia levels, lower DO, and the presence of a foul odor in the water indicated the decomposition of organic matter, which likely triggered acute mortality in C. striata. Similarly, Attia et al. (2022) reported that poor water quality, including lower DO and elevated ammonia, combined with parasitic and bacterial pathogens intensify the fish mortalities. Moreover, low water quality, including a higher organic load, has been identified as a key factor contributing to the outbreak of parasitic infections in fish (Mamun et al., 2020). Parasitic diseases pose a significant threat in traditional pond aquaculture system of Bangladesh. The major parasitic groups of fish include, protozoans, trematodes, cestodes, annelids and copepodes causing frequent disease outbreak both in wild and cultured stock (Madsen and Stauffer, 2024). Numerous documentation has been reported of trematodes infestation in Channa spp. (Das et al., 2018). However, limited reports are available on infection of cercariae, a juvenile stage of digenean parasites. The lifecycle of digenean parasites involves multiple hosts, starting with eggs that hatch into miracidia, infecting a snail (first intermediate host) where they develop into cercariae, which then infect fish or amphibians (second intermediate host) before maturing into adults in the definitive host, typically a bird or mammal (Cribb et al., 2014). Fish often serve as intermediate hosts for trematode cercariae and different organs are the sites of penetration (Mikheev et al., 2014). Metacercariae are more commonly found in fish compared to cercariae or adult trematodes (Noga, 2014). Cercariae invade fish hosts, migrating to target organs and causing damage such as hemorrhaging, necrosis, and inflammation along their path (Morley, 2020), which is also evident in our study. The mass cercarial infection in C. striata caused severe skin abrasions, deep ulcerations, and extensive tissue necrosis, resulting from the significant damage inflicted by the penetration and migration of cercariae. Heavy, acute infections of cercariae can be fatal, especially to small fish (Mikheev et al., 2014). The current study revealed a higher infestation rate in older and larger fish, with those weighing ≥50 g exhibiting an 83% prevalence compared to 75% in smaller fish weighing ≤50 g. In contrast, Mamun et al. (2016) reported the highest infection rates in medium-sized fish, while larger fish showed the lowest prevalence of infections. Parasitic and bacterial coinfections frequently occur in aquaculture, especially under intensive or controlled environmental conditions (Okon et al., 2023). These coinfections often intensify disease dynamics and severity, with each pathogen has different pathognomonic features while coexisting with others, ultimately resulting in increased fish mortality (Attia et al., 2022; Okon et al., 2023). In the current study the coinfections of A. veronii and trematodes cercariae infections caused up to 80% mortality in Ayesha Poultry and Fishery Bohor, Gowainghat, Sylhet (owner’s observation). A recent study reported mass mortality of farmed common carp (Cyprinus carpio) in Egypt, attributed to the dual infection of Dactylogyrus extensus and Pseudomonas fluorescens (Attia et al., 2022). Numerous studies have reported coinfections in fish involving parasites and bacteria, which significantly enhance mortality rates (Kotob et al., 2017; Wise et al., 2021; Abdel‐Latif et al., 2020). Histopathological examinations in our present study revealed systemic abnormalities, including inflammation, congestion, and necrosis across multiple organs of C. striata. Similar findings were documented in C. punctata, where trematode infestations caused significant immune cell infiltration and tissue damage in the skin and muscle (Bari et al., 2024a). Moreover, severe histopathological changes, such as the loss of secondary gill lamellae and the occurrence of cellular hypertrophy and hyperplasia, have been reported in fish infected with the protozoan parasite Ichthyophthirius multifiliis (Mamun et al., 2020; Mamun et al., 2021b), are also evident in our study. The genus Aeromonas, belonging to the family Aeromonadaceae, comprises Gram-negative bacteria widely distributed in aquatic environments, with several species capable of causing diseases in humans, fish, and other aquatic animals (Fernández-Bravo et al., 2020; Goni et al., 2020; Parven et al., 2020). Among Aeromonas spp., an increasing incidence of A. veronii outbreaks has been observed, with this bacterium being isolated and identified through various bacteriological and molecular techniques from commercial aquaculture farms in Bangladesh (Ehsan et al., 2023; Bari et al., 2024b). Moreover, very recently, Mawa et al. (2024) reported mass mortality of Gangetic Mystus (Mystus cavasius) in the biofloc aquaculture system from the Brahmanbaria district of Bangladesh. This study is the first to report a coinfections of bacterial and parasitic pathogens occurring in a biofloc system in Bangladesh. Striped snakehead fingerlings (C. striata) experienced over 70% mortality due to A. veronii infection in Malaysia (Tahir et al., 2024). The chances of disease outbreaks are higher in biofloc systems compared to traditional aquaculture systems due to factors such as higher stocking densities, accumulation of unused flocs, and fluctuations in water quality parameters (Raza et al., 2024). Biofloc technology has emerged as an innovative and promising technique for disease control, promoting antibiotic-free aquaculture systems (Raza et al., 2024). However, our survey data reveal that farmers in biofloc aquaculture systems often use antibiotics indiscriminately, without identifying the specific antibiotic effective against particular bacteria. This excessive and improper use of antibiotics has significantly contributed to the emergence of antibiotic-resistant bacteria in aquaculture. The present study demonstrated that A. veronii exhibited resistance to multiple antibiotics, underscoring the urgent need for sustainable and alternative approaches to disease management.
5. Conclusions
Biofloc technology based aquaculture system can potentially serve as sustainable aquatic crop production and contribute to achieve the sustainable development goals (SDGs). Although this technology has been proven successful across the world aquaculture system, however frequent disease outbreak and limitations in continuous power supply and quality fry/spawns availability has raised serious question on its success and sustainability. It is not surprising to outline that biofloc aquaculture system become bio-flop system in Bangladesh as our result evident that 75% of the respondent opined biofloc as a non-sustainable technology. Moreover, our survey revealed that most farmers use antibiotics indiscriminately, without proper diagnosis or antibiogram study. This practice poses significant risks, including environmental contamination, endangerment of aquatic fauna, accumulation of residues, and the development of antibiotic-resistant bacteria, which could lead to catastrophic outcomes. In many cases, farmers could not manage the culture system when there is power outages and disease outbreak allowing entire crop to die. The virulent pathogen A. veronii along with trematodes cercariae infection in C. striata resulted severe mortality in one of the biofloc farm indicating the challenges and non-sustainability of biofloc technology in Bangladesh. However, further studies are essential to comprehensively analyze the sustainability of biofloc technology in Bangladesh, focusing on overcoming its current challenges and optimizing its potential for long-term success in aquaculture.
Acknowledgements
This work was conducted under the project entitled “Investigation on various fish diseases and interactive factors causing disease incidences in several biofloc farms of Sylhet district.” We extend our heartfelt gratitude to the biofloc farmers who generously participated in our study by sharing valuable insights during the questionnaire interviews. Additionally, we are deeply thankful to the owner of Ayesha Poultry and Fishery for providing the valuable fish specimens for the investigations. We express our heartfelt gratitude to the Sylhet Agricultural University Research System (SAURES), Sylhet Agricultural University, Sylhet-3100, Bangladesh, for their financial support under the project ID SAURES-UGC-22-23-69; Memo No-SAU/Direc.(Research)-128/22/592(27).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Informed consent statement
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contribution
Conceptualization: Md. Abdullah Al Mamun, Data collection: Anupoma Achariya, Siddikur Rahman Sujon, Data analysis: Shamima Nasren, M. M. Mahbub Alam, Figure preparation: Md. Abdullah Al Mamun and Sarker Mohammed Ibrahim Khalil. All authors critically reviewed the manuscript and agreed to submit final version of the manuscript



