Pakistan is among the world's most populous nations. Food insecurity affects 47% of Pakistan's poeple, and there is a widespread protein shortage and unequal access to food (Mujahid et al., 2022 ![]() ; Abdul and Abdul, 2020
; Abdul and Abdul, 2020 ![]() ). Malnutrition can be exacerbated by the high cost of a healthy diet, particularly for impoverished households (Felipe et al., 2019
). Malnutrition can be exacerbated by the high cost of a healthy diet, particularly for impoverished households (Felipe et al., 2019 ![]() ). To address this problem, the Pakistani government should develop more diverse protein sources for people of all ages (Sajid, 2022
). To address this problem, the Pakistani government should develop more diverse protein sources for people of all ages (Sajid, 2022 ![]() ). The artificial breeding of a variety of fishes under hatchery conditions is a promising approach to meet protein requirements in developing countries such as Pakistan (Muhammad, 2018
). The artificial breeding of a variety of fishes under hatchery conditions is a promising approach to meet protein requirements in developing countries such as Pakistan (Muhammad, 2018 ![]() ).
).
Pangasius hypophthalmus (Sauvage, 1878) is a stripped catfish that is a member of the Pangasiidae family of the order Siluriformes (Sajid, 2022 ![]() ). This pangasiid catfish species is highly significant in the Mekong River in Southeast Asia (An et al., 2022
). This pangasiid catfish species is highly significant in the Mekong River in Southeast Asia (An et al., 2022 ![]() ; Nguyen et al., 2022
; Nguyen et al., 2022 ![]() ). They exhibit omnivorous feeding behavior, faster growth rates, lower production costs, and high-stress tolerance capacities and can withstand a wide range of environmental factors (Chowdhury et al., 2020
). They exhibit omnivorous feeding behavior, faster growth rates, lower production costs, and high-stress tolerance capacities and can withstand a wide range of environmental factors (Chowdhury et al., 2020 ![]() ; Afiya et al., 2019
; Afiya et al., 2019 ![]() ). Many Asian nations, including Vietnam, Bangladesh, China, Indonesia, Malaysia, Laos, and Cambodia, are major producers, whereas Vietnam is at the top in farming and export of this fish (Tram Anh et al., 2023
). Many Asian nations, including Vietnam, Bangladesh, China, Indonesia, Malaysia, Laos, and Cambodia, are major producers, whereas Vietnam is at the top in farming and export of this fish (Tram Anh et al., 2023 ![]() ). The reported natural breeding season is between June and August. It has been reported that it can be grown well for monoculture and polyculture with other carps in hatchery environments (Rahman et al., 2020
). The reported natural breeding season is between June and August. It has been reported that it can be grown well for monoculture and polyculture with other carps in hatchery environments (Rahman et al., 2020 ![]() ). It can be sexually mature at the age of 3–3.5 years after it gains 3 kg of weight but can reach up to 15 kg of weight. They are highly fecund, as a mature female fish of 10 kg can produce one million eggs (Abdallah et al., 2021
). It can be sexually mature at the age of 3–3.5 years after it gains 3 kg of weight but can reach up to 15 kg of weight. They are highly fecund, as a mature female fish of 10 kg can produce one million eggs (Abdallah et al., 2021 ![]() ). Its meat offers nutritious fats like omega-3 fatty acids. Additionally, it contains 60% and 72% of crude protein by weight (Alphonse et al., 2020
). Its meat offers nutritious fats like omega-3 fatty acids. Additionally, it contains 60% and 72% of crude protein by weight (Alphonse et al., 2020 ![]() ).
).
Some carnivorous teleost species do not ovulate or spermiate while in culture due to a lack of natural environmental stimulus, stress, or inhibition of gonadotropin-releasing hormone (GnRH) release by the fish pituitary gland via the action of dopamine (Radha, 2023 ![]() ). Pituitary gonadotropins (follicle-stimulating hormone and luteinizing hormone) are responsible for sex hormone production and gametes release in both male and female fish, and their secretion is stimulated by GnRH (José Antonio et al., 2020
). Pituitary gonadotropins (follicle-stimulating hormone and luteinizing hormone) are responsible for sex hormone production and gametes release in both male and female fish, and their secretion is stimulated by GnRH (José Antonio et al., 2020 ![]() ). Dopamine blocks dopamine type-2 (DA2) receptors present in the pituitary and thus inhibits the ability of GnRH to stimulate FSH and LH release. Hence, artificially induced fish spawning is achieved via the use of various drugs, such as ovaprim (OVP), human chorionic gonadotropin (HCG), carp pituitary extract (CPE), ovaplant and ovatide (Amirah et al., 2022
). Dopamine blocks dopamine type-2 (DA2) receptors present in the pituitary and thus inhibits the ability of GnRH to stimulate FSH and LH release. Hence, artificially induced fish spawning is achieved via the use of various drugs, such as ovaprim (OVP), human chorionic gonadotropin (HCG), carp pituitary extract (CPE), ovaplant and ovatide (Amirah et al., 2022 ![]() ). OVP, an artificial gametogenic and spawning drug,that can be administered to sexually mature fishes in captivity intramuscularly or intraperitoneally. Compared with other exogenous hormones, such as spawnprim and HCG, OVP provides better reproductive results in P. hypophthalmus (Vinka et al., 2021
). OVP, an artificial gametogenic and spawning drug,that can be administered to sexually mature fishes in captivity intramuscularly or intraperitoneally. Compared with other exogenous hormones, such as spawnprim and HCG, OVP provides better reproductive results in P. hypophthalmus (Vinka et al., 2021 ![]() ).
).
The main aim of this research was to determine the optimal dosage of ovaprim for increasing the ovulation rate in female P. hypophthalmus and to investigate its impact on the latency period. Furthermore, the objective of this study was to accomplish effective reproduction under different climatic conditions of Pakistan to optimize seed production and improve the availability of P. hypophthalmus for local consumption. The research includes a concise assessment of the relevant literature and references to support its context and significance. This research provides valuable information on the most effective dosage of ovaprim for enhancing ovulation in P. hypophthalmus hence improving seed output and promoting the long-term viability of the aquaculture business in Pakistan. This study enhances breeding success rates, hence increasing the accessibility of P. hypophthalmus for local consumption.
2. Materials and Methods
2.1 Ethical approval
No ethical approval is required for this study.
2.2 Managing and selecting broodstocks for induced spawning
Broodstock was moved from the Fish Farm Complex of the Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Pattoki, Pakistan, to the Fish Hatchery Balloki (Sindhwan) District Kasur (Figure 1).
They were acclimatized in 30 L of 55 L capacity cement tanks for 48 h. Broodstocks of P. hypophthalmus included sexually mature male and female fish aged two and three years, respectively (Figure 2). These were fed 30% proteinous feed @ 3% live fish b.w. The ethical requirements of the University of Veterinary and Animal Sciences, Pakistan, adhered to the processes and methods employed in the study.

With the use of a seine net, the adult male and female were removed from the pond and carefully moved into the hatchery building's tanks. The males and females were then placed individually in, the breeding tank. Ripe fish were chosen for breeding based on an assessment of their abdomen, genital aperture, and pectoral fin, both visually and physically (Subhendu and Ferosekhan, 2018 ![]() ). Females with a noticeable belly bulge and a pinkish-colored vent filled with eggs were chosen, while males who oozed milt when their abdomen was compressed were chosen. These broodfish were placed in a circular tank with a continuous supply of oxygenated water for eight hours for conditioning. Six pairs of broodfish were selected for induction of OVP injection to observe the ovulation and latency periods of P. hypophthalmus. The first group consisted of females weighing between 3.5 and 3.7 kg and males weighing between 4.2 and 4.5 kg. The second group consisted of females weighing between 3.8 and 4.1 kg and males weighing 4.03 to 4.1 kg. The third group consisted of females weighing between 5.47 and 5.5 kg and males weighing between 4.7 and 4.8 kg.
). Females with a noticeable belly bulge and a pinkish-colored vent filled with eggs were chosen, while males who oozed milt when their abdomen was compressed were chosen. These broodfish were placed in a circular tank with a continuous supply of oxygenated water for eight hours for conditioning. Six pairs of broodfish were selected for induction of OVP injection to observe the ovulation and latency periods of P. hypophthalmus. The first group consisted of females weighing between 3.5 and 3.7 kg and males weighing between 4.2 and 4.5 kg. The second group consisted of females weighing between 3.8 and 4.1 kg and males weighing 4.03 to 4.1 kg. The third group consisted of females weighing between 5.47 and 5.5 kg and males weighing between 4.7 and 4.8 kg.
2.3 Hormonal administration
This experiment was conducted in three separate tanks, each containing two pairs of fish, and this study involved the testing of four doses of OVP (Figure 2). One group of females was administered 0.5 ml/kg OVP, a dose of OVP for hormone injection; the second group of females was given 0.6 ml/kg hormone; the third group was given 0.7 ml/kg OVP b.w.; and the males in all three groups were given OVP injections @ 0.2 ml/kg b.w. intramuscularly below their dorsal fin.
As soon as the hormone was given, the brooders were placed in their individual tanks. The temperature was held constant at 28–30 °C, the dissolved O₂ level was 5.5–6.0 mg/L, the salinity was 0.9 ppt, and the pH was 7.9–8.2 throughout the trial. Up to ovulation, spawning behaviors and behavioral changes associated with breeding were noted.
2.4 Stripping and fertilization
Gravid female fish were stripped by slightly pressing the abdomen manually to collect their eggs in a fertilization tray. After the eggs were placed on the tray, the males were also promptly stripped to obtain their milt and placed in the same tray (Figure 3). The process of fertilizing eggs involved combining them with milt and allowing them to stand for three to five minutes. Fresh water was also added, and the mixture was stirred continuously for 5–6 min to activate the sperm and maximize the fertilization rate. After this, the whole mixture was mixed well with Multani Mitti to remove the outer adhesive coatings of P. hypophthalmus eggs, thus preventing egg agglutination and enhancing seed production. Hence, Multani Mitti is the best choice for the degumming of P. hypophthalmus eggs.

2.5 Incubation period and egg hatching
Multani Mitti was removed from the fertilized eggs by repeatedly washing them in freshwater. A continuous water circulation system was used to move the enlarge shells in different hatching jars. Throughout the incubation period, the jar's water flow was adjusted to 600–800 ml/min. After 22–25 h of fertilization, hatching was completed within 2.0–4.0 h at temperatures ranging from 26–31 °C (Figure 4). Within an hour after hatching, unfertilized eggs and eggshells were taken out of the hatchling jar to prevent fungal infection in the larvae. The fine egg yolk emulsion was then combined with water to nourish the hatchling after it had hatched. After being removed from jars, larvae from various parent pairs were placed in nursery ponds that had been set up before. Throughout the experiment, the temperature of the water was noted.

2.6 Statistical analysis
One- and two-way ANOVA was used to statistically analyze the data, taking into consideration the effects of the latency period and ovaprim dosage. The research was carried out using SPSS-20 for Windows, and means were compared at a significance level of P<0.05 using Duncan's novel multiple range tests. The average value means ± standard errors of the means (SEM) are used to show the results.
3. Results
3.1 Effects of OVP on the latency period and ovulation
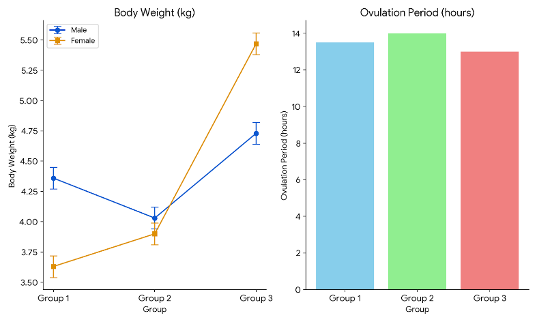
The outcome of OVP treatment was successful, as all P. hypophthalmus (n = 12) artificially spawned and produced viable gametes in captivity. The latency period was different among female fish because of the different OVP doses used (Table 1). It was observed between 18–16 h after OVP administration. The shortest latency period (16 h) was observed in females in the third group, which were given an OVP dose of 0.7 ml/kg b.w. The longest latency period was recorded in the first group of females after the administration of 0.5 ml/kg OVP.
Table 1. The latency period (hr.) and percentage of egg fertilization.
OVP injections of 0.5, 0.6, and 0.7 ml/kg b.w. successfully induced ovulation in all female P. hypophthalmus. In all females, the ovulation period ranged between 12 and 15 h after OVP hormonal injection. At the OVP dose of 0.7 ml/kg, the best ovulation period (12–14 h) was observed in the third group, with a maximum fertilization rate (approx. 65%). The ovulated eggs were of good quality. Hence, the dose of 0.7 ml/kg OVP had the best results, as shown in Figure 5.
3.2 Egg and embryonic development
Before fertilization, the each ova's diameter varied from 1.0 to 1.5 mm. The fertilized eggs were translucent, spherical, and had a sticky quality. Following fertilization and water hardening, the diameter of the eggs expanded to about 0.2 mm. Throughout the developing stage, the water's temperature was kept between 26 and 29 °C. After 18–24 hours of fertilization at 27–29 °C, hatchlings appeared. The percentage of eggs that hatch varies and can be anywhere from 20 and 80%, contingent upon the quality and level of fertilization.
4. Discussion
Our study revealed that a single dose of OVP (0.7 ml/kg b.w.) effectively induced ovulation in P. hypophthalmus. It was concluded that OVP may be used by hatchery operators to artificially promote their maximum spawning. This study evaluated the effects of different doses of OVP on the latency period and ovulation of P. hypophthalmus.
The current study reported the successful artificial breeding of P. hypophthalmus in the agroclimatic conditions of Head Balloki Fish Hatchery (Sindhwan) via OVP administration, which improved fry and fingerling survival. A key element in the success of a breeding program is the selection of the best breeders who are in good health for the production of high-quality gametes (Prem et al., 2021 ![]() ). A good-quality broodstock, which was originally obtained from Thailand, was selected for this study. Through the use of OVP, P. hypophthalmus was successfully induced to spawn and produce seeds (10, 50,000 fry and 6, 30,000 fingerlings) in Sindhwan. Approximately 0.8 to 1.5 lakh eggs were released per female during the stripping process, possibly because this species only spawns once a season. Similar findings on the fecundity of P. hypophthalmus were reported by Subhendu and Ferosekhan (2018
). A good-quality broodstock, which was originally obtained from Thailand, was selected for this study. Through the use of OVP, P. hypophthalmus was successfully induced to spawn and produce seeds (10, 50,000 fry and 6, 30,000 fingerlings) in Sindhwan. Approximately 0.8 to 1.5 lakh eggs were released per female during the stripping process, possibly because this species only spawns once a season. Similar findings on the fecundity of P. hypophthalmus were reported by Subhendu and Ferosekhan (2018 ![]() ).
).
Compared with the 0.5 and 0.6 ml/kg doses, OVP at a 0.7 ml/kg dose in female P. hypophthalmus resulted in the shortest ovulation (12–14 h) and latency period (16 h), with a maximum fertilization rate (approx. 65%). All females in this study needed a minimum of 13 and a maximum of 15 h to ovulate after OVP injection. Several studies have previously reported the ovulation-inducing capability of OVP in this catfish and in other fish species (Erfan et al., 2023 ![]() ; Sah et al., 2018
; Sah et al., 2018 ![]() ; Muhammad et al., 2021
; Muhammad et al., 2021 ![]() ). The mechanism of action of OVP involves the release of pituitary gonadotropins in response to salmon gonadotropin-releasing hormone (sGnRH) in the OVP. However, domperidone (a dopamine inhibitor), which is also the main component of OVP, further inhibits natural GnRH release. Hence, in sexually mature fish, the release of stored pituitary gonadotropins facilitates ovulation and spermiation (Yoshitaka, 2023
). The mechanism of action of OVP involves the release of pituitary gonadotropins in response to salmon gonadotropin-releasing hormone (sGnRH) in the OVP. However, domperidone (a dopamine inhibitor), which is also the main component of OVP, further inhibits natural GnRH release. Hence, in sexually mature fish, the release of stored pituitary gonadotropins facilitates ovulation and spermiation (Yoshitaka, 2023 ![]() ; Vahid et al., 2018
; Vahid et al., 2018 ![]() ). These results indicate that OVP can potentially induce gametogenesis and spawning artificially in P. hypophthalmus, resulting in a short ovulation and latency period.
). These results indicate that OVP can potentially induce gametogenesis and spawning artificially in P. hypophthalmus, resulting in a short ovulation and latency period.
The optimal spawning happened in June at four different OVP dosages, i.e., 0.5, 0.6, and 0.7 ml/kg OVP b.w. in females and 0.2 ml/kg in male fish. After a single injection, the OVP optimum dose to trigger spawning in female P. hypophthalmus has been shown to be 0.60–0.70 ml/kg b.w. These dosages are almost similar to those used in Labeo rohita, Cirrhinus cirrhosus, and Catla catla breeding procedures (i.e. 0.40–0.50 ml, 0.30–0.40 ml, and 0.25–0.30 ml/kg b.w., respectively). A dose of 0.60 ml/kg or 0.70 ml/kg b.w. resulted in good fertilization, latency periods, hatching, and ovulation rates, in P. hypophthalmus.
In general, an incubation period of 18 to 24 h is provided for successful egg hatching, and these results are in line with those of a previous study (Binay, 2021 ![]() ), where, 13–15 h after 0.6 ml/kg b.w. OVP injection, ovulation occurred, and hatching occurred 18–24 h following fertilization. Their mature unfertilized eggs are elastic and spherical, while mature fertilized ones are round and sticky. The yolk spheres and egg capsules have a greenish-brown color. Fish from the same catfish family (e.g., Clarias batrachus) have a greenish egg capsule (Bilal et al., 2014). After the fertilized eggs were incubated in the hatchery, P. hypophthalmus further increased in size by approximately 0.2 mm, which may have been due to egg hydration. The fertilized eggs strongly adhered and were located in the clutch between eggs during egg incubation in the hatchery. During the hatchery's egg incubation process, the fertilized eggs were found in the clutch and adhered firmly. The temperature during the trial period was reported in the range of 27.2–29.6 °C. Abdus et al. (2013
), where, 13–15 h after 0.6 ml/kg b.w. OVP injection, ovulation occurred, and hatching occurred 18–24 h following fertilization. Their mature unfertilized eggs are elastic and spherical, while mature fertilized ones are round and sticky. The yolk spheres and egg capsules have a greenish-brown color. Fish from the same catfish family (e.g., Clarias batrachus) have a greenish egg capsule (Bilal et al., 2014). After the fertilized eggs were incubated in the hatchery, P. hypophthalmus further increased in size by approximately 0.2 mm, which may have been due to egg hydration. The fertilized eggs strongly adhered and were located in the clutch between eggs during egg incubation in the hatchery. During the hatchery's egg incubation process, the fertilized eggs were found in the clutch and adhered firmly. The temperature during the trial period was reported in the range of 27.2–29.6 °C. Abdus et al. (2013 ![]() ) noted that the primary controlling factor for ovulation and hatchling was water temperature.
) noted that the primary controlling factor for ovulation and hatchling was water temperature.
It can be easily cultured in Pakistan, but the primary obstacle to its cultivation is the limited supply of its seed. Hence, seed production is a limiting factor for increasing yield. To overcome this problem, it is necessary to increase our country's seed production by improving its breeding technique.
5. Conclusions
The results of our research demonstrated that the administration of a single dose of ovaprim (0.7 ml/kg b.w.) successfully stimulated ovulation in P. hypophthalmus. This finding establishes ovaprim as a practical choice for hatchery operators seeking to optimize artificial spawning. This study assessed the effects of various dosages of OVP on the time it takes for ovulation to occur and the rates of ovulation to determine the most effective dose for real-world use. Although this study offered valuable information, more research is necessary to investigate the long-term impacts and improve the methods of administering the dosage. Future research should prioritize the optimization of environmental circumstances and the exploration of genetic elements to improve the reproductive success of P. hypophthalmus.
Acknowledgements
The authors acknowledge Mr. Muhammad Ashraf, Assistant Director, and staff members of Fish Hatchery Balloki (Sindhwan) District Kasur, Pakistan, for providing the lab facilities.
Data availability statement
Not applicable.
Informed consent statement
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
Conceptualization: MSS; Data collection: SA and FK; Data analysis: IM and HM; Writing, review, and editing: FK; Figure preparation and Writing, review, and editing: SHAS. All the authors critically reviewed the manuscript and agreed to submit a final version of the article



