Molecular detection and antibiogram profiles of Escherichia coli and Vibrio cholerae isolated from raw vegetables in the northern district of Bangladesh
Show More
1 Department of Microbiology, Hajee Mohammed Danesh Science and Technology University, Dinajpur-5200, Bangladesh
2 Department of Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh
3 Department of Biotechnology, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh
4 Department of Internal Medicine, Shimane University, 89-1 Enyacho, Izumo, Shimane 693-0021, Japan
5 Department of Microbiology, Gono Bishwabidyalay, Savar, Dhaka-1344, Bangladesh
*Corresponding author:
Email address: aoulad1992@gmail.com (Md. Aoulad Hosen)
Share:
Received:
01 June 2024
Revised:
31 July 2024
Accepted:
26 August 2024
Published:
30 August 2024
Highlights
- Identified E. coli and V. cholerae in raw vegetables, highlighting contamination risks in food safety practices.
- Employed PCR for precise molecular identification of pathogens, enhancing diagnostic capabilities in food microbiology.
- Characterized antimicrobial resistance and multidrug resistance (MDR) profiles of isolated strains, emphasizing public health implications.
- Demonstrated the importance of stringent food safety measures and antimicrobial stewardship in agricultural settings.
- Contributed essential insights into mitigating agricultural contamination risks, crucial for public health management.
Abstract
Raw vegetables are essential for a well-balanced diet as they provide vitamins, minerals, dietary fiber, and phytochemicals. This study aimed to isolate, identify, and evaluate the microbial loads of Escherichia coli and Vibrio cholerae in raw vegetables sold at local markets in the Dinajpur district of Bangladesh. A total of 35 vegetable samples were collected from four markets in Dinajpur district. The isolates were identified using cultural, staining, biochemical, and molecular tests. Microbial loads were enumerated (TVC) using the pour plate technique. Molecular detection of bacterial species was confirmed targeting the 16S rRNA and groEL genes of E. coli and V. cholerae, respectively. The amplification was done on 704 bp fragments of the 16S rRNA gene of E. coli and 1117 bp fragments of Vibrio spp. For the confirmation of V. cholerae, amplification of a 418 bp fragment of the groEL gene was performed through multiplex PCR. An antimicrobial susceptibility test was conducted on all isolates of bacteria against eleven and eight antibiotics by disc diffusion. The total viable count (TVC) in potato, carrot, cabbage, cauliflower, tomato, green chili, cucumber, mustard sak, and coriander leaves were 2.4 ± 0.37, 2.2±0.14, 2.1±0.26, 1.8±0.14, 1.7±0.27, 1.5±0.33, 1.5±0.33, and 1.4±0.25 mean log colony forming units ± standard deviation/mg, respectively. Out of 35 raw vegetable samples, 16 (45.71%) and 13 (37.14%) isolates were culture positive for E. coli and V. cholerae. Subsequently, 5 (31.25%) and 4 (30.76%) isolates of E. coli and V. choleraewere confirmed positive molecularly. All 16 and 13 isolates of E. coli and V. cholerae were subjected to antibiogram testing against 11 and 8 antibiotics. E. coli isolates were highly resistant to ceftazidime, cefixime, ampicillin, and oxytetracycline, but sensitive to gentamycin, ceftriaxone, colistin, and enrofloxacin. Similarly, V. cholerae isolates were highly resistant to nalidixic acid, trimethoprim, and polymyxin, but highly sensitive to kanamycin, gentamicin, and streptomycin. The study’s findings indicate that raw vegetables pose a significant public health risk due to MDR E. coli and V. cholerae. To achieve safer levels of these bacteria in raw vegetables, good production practices and hygiene awareness are essential.
Graphical abstract
Keywords
1. Introduction
Raw vegetables make up a significant portion of a person's diet in many parts of the world and are beneficial to nutrition, especially in terms of phyto-nutrieuticals, which include minerals, dietary fiber, vitamins C, A, B1, B6, B9, and E, and phytochemicals (Silva Dias, 2022 ![]() ). Consuming vegetables on a daily basis has been linked to improved gastrointestinal health, clear vision, and a decreased risk of heart disease, stroke, chronic illnesses including diabetes, and multiple cancers. Vegetables contain powerful antioxidant phytochemicals that can reduce the risk of developing chronic illnesses through preventing damage from free radicals, altering the metabolic and detoxification processes of carcinogens, or even enacting mechanisms that alter the growth of tumor cells (Rudzińska et al., 2023
). Consuming vegetables on a daily basis has been linked to improved gastrointestinal health, clear vision, and a decreased risk of heart disease, stroke, chronic illnesses including diabetes, and multiple cancers. Vegetables contain powerful antioxidant phytochemicals that can reduce the risk of developing chronic illnesses through preventing damage from free radicals, altering the metabolic and detoxification processes of carcinogens, or even enacting mechanisms that alter the growth of tumor cells (Rudzińska et al., 2023 ![]() ; Pinto et al., 2021
; Pinto et al., 2021 ![]() ; Sotler et al., 2019
; Sotler et al., 2019 ![]() ; Dias, 2012
; Dias, 2012 ![]() ).
).
In Bangladesh total vegetable consumption in 2013 was 4,049 kt, according to Faostat (Haque and Hoque, 2021 ![]() ). This represents a 0.226% increase over the prior year. This is 0.226 percent higher than it was the year before (Aalm and Nase, 2020
). This represents a 0.226% increase over the prior year. This is 0.226 percent higher than it was the year before (Aalm and Nase, 2020 ![]() ).
).
Despite their many health advantages, eating fresh vegetables has also been linked to risks for consumers (Bekele et al., 2017 ![]() ). Vegetables are typically eaten raw because they are high in fiber, vitamins, minerals, antioxidants, and carbs. Phytonutrients have the potential to function as efficient carriers of infections (Desiree et al., 2021
). Vegetables are typically eaten raw because they are high in fiber, vitamins, minerals, antioxidants, and carbs. Phytonutrients have the potential to function as efficient carriers of infections (Desiree et al., 2021 ![]() ; Said, 2012
; Said, 2012 ![]() ). Pathogenic microorganisms can contaminate raw vegetables when they are grown in fields, harvested, handled after harvest, processed, and distributed. Vegetables can become contaminated at different times by different agronomic techniques. Most contamination occurs before harvesting, either directly from domestic and wild animals or through the use of sewage, irrigation water, effluent from livestock operations, or contaminated manure. It can also happen during the harvesting, transport, processing, distribution, and marketing processes, or even at home (Alegbeleye et al., 2023
). Pathogenic microorganisms can contaminate raw vegetables when they are grown in fields, harvested, handled after harvest, processed, and distributed. Vegetables can become contaminated at different times by different agronomic techniques. Most contamination occurs before harvesting, either directly from domestic and wild animals or through the use of sewage, irrigation water, effluent from livestock operations, or contaminated manure. It can also happen during the harvesting, transport, processing, distribution, and marketing processes, or even at home (Alegbeleye et al., 2023 ![]() ; Łepecka et al., 2022
; Łepecka et al., 2022 ![]() ). The quality of raw vegetables and human health are impacted when wastewater is used for irrigation. It might be the potential source of harmful microbes on vegetables. Bacteria such as Escherichia coli O157:H7, Shigella spp., Vibrio spp., Campylobacter spp., L. monocytogenes, and Salmonella spp. (Osafo et al., 2022
). The quality of raw vegetables and human health are impacted when wastewater is used for irrigation. It might be the potential source of harmful microbes on vegetables. Bacteria such as Escherichia coli O157:H7, Shigella spp., Vibrio spp., Campylobacter spp., L. monocytogenes, and Salmonella spp. (Osafo et al., 2022 ![]() ). It can infect raw vegetables when it comes into touch with dung and sewage. In addition, contaminations can happen after harvest through unclean wash water, cross-contamination from an infected food handler, and eating of contaminated food, whether raw or cooked, can be a significant risk factor for the spread of pathogens (Machado‐Moreira et al., 2019
). It can infect raw vegetables when it comes into touch with dung and sewage. In addition, contaminations can happen after harvest through unclean wash water, cross-contamination from an infected food handler, and eating of contaminated food, whether raw or cooked, can be a significant risk factor for the spread of pathogens (Machado‐Moreira et al., 2019 ![]() ; Said, 2012
; Said, 2012 ![]() ). Contamination and growth of spoilage microorganisms usually limit the shelf life of vegetables (Alegbeleye et al., 2022
). Contamination and growth of spoilage microorganisms usually limit the shelf life of vegetables (Alegbeleye et al., 2022 ![]() ). Food-borne illnesses are caused by a variety of microbe types and the toxins they produce, including Salmonella spp., Shigella spp., E. coli, Bacillus anthracis, Klebsiella spp., Brucella spp., L. monocytogenes, Yersenia enterolytica, and Eubacteriaceae (Islam et al., 2023
). Food-borne illnesses are caused by a variety of microbe types and the toxins they produce, including Salmonella spp., Shigella spp., E. coli, Bacillus anthracis, Klebsiella spp., Brucella spp., L. monocytogenes, Yersenia enterolytica, and Eubacteriaceae (Islam et al., 2023 ![]() ; Julqarnain et al., 2022
; Julqarnain et al., 2022 ![]() ).
).
E. coli may be a common facultative resident of the human environment and the large intestine. It is one of the most prevalent causes of a number of common bacterial infections, including as newborn meningitis, cholecystitis, bacteremia, cholangitis, tract infections (UTI), traveler's diarrhea, and other clinical illnesses (Hossain et al., 2024 ![]() ; Ema et al., 2022
; Ema et al., 2022 ![]() ). E. coli spreads by the oral and fecal routes. It is frequently found in food, soil, and water due to its adaptability and versatility. The potential of bacterial contamination is very significant when using raw manure as fertilizer (Ongeng et al., 2015
). E. coli spreads by the oral and fecal routes. It is frequently found in food, soil, and water due to its adaptability and versatility. The potential of bacterial contamination is very significant when using raw manure as fertilizer (Ongeng et al., 2015 ![]() ).
).
V. cholerae is a gram-negative, highly motile, curved or comma-shaped rod bacterium that generates cholerae enterotoxin and causes life-threatening secretory diarrhea (Weil and Harris, 2015 ![]() ). Usually, the bacteria are found in freshwater lakes and rivers and other aquatic habitats. The most common way that cholera spreads to humans or animals is through contaminated water sources (Abioye et al., 2021
). Usually, the bacteria are found in freshwater lakes and rivers and other aquatic habitats. The most common way that cholera spreads to humans or animals is through contaminated water sources (Abioye et al., 2021 ![]() ). The source of contamination is the feces of diseased people. In humans and certain animals, cholera can result in severe diarrhea, vomiting, dehydration, and shock. Death may come within hours if treatment is not received (Chowdhury et al., 2022
). The source of contamination is the feces of diseased people. In humans and certain animals, cholera can result in severe diarrhea, vomiting, dehydration, and shock. Death may come within hours if treatment is not received (Chowdhury et al., 2022 ![]() ). Man may consume the germs and become exposed to it (Baker-Austin et al., 2018
). Man may consume the germs and become exposed to it (Baker-Austin et al., 2018 ![]() ). This could happen from drinking contaminated water, eating raw vegetables, or coming into contact with the excrement of diseased animals or humans (Rebaudet et al., 2013
). This could happen from drinking contaminated water, eating raw vegetables, or coming into contact with the excrement of diseased animals or humans (Rebaudet et al., 2013 ![]() ).
).
E. coli bacteria can be found in food, the environment, and the intestines of both people and animals. E. coli is a very large type of bacteria. Most strains of E. coli are toxic, while some can make you unsound. While some strains of E. coli can cause pneumonia, lung illnesses, tract infections, and other illnesses, others can cause diarrhea (Fleckenstein and Kuhlmann, 2019 ![]() ).
).
E. coli and V. cholerae have been documented to be responsible for outbreaks connected to the intake of fresh but raw vegetables and fruits (such as lettuce, spinach, carrots) (Aurin et al., 2020 ![]() ). Pathogenic E. coli and V. cholerae can infect raw vegetables when they are growing in the field, when fertilizer (cow dung) is applied, or when the veggies are harvested, transported, processed, stored, and distributed. Thus far, no reports of outbreaks or sporadic illnesses linked to V. cholerae and E. coli from fresh raw vegetables have come from Bangladesh. In order to isolate E. coli and V. cholerae raw vegetables were gathered near Dinajpur city for this purpose. Therefore, this study was conducted to isolate, identify, and evaluate the microbial loads along with the antimicrobial susceptibility patterns of E. coli and V. cholerae in raw vegetables sold in local markets in the Dinajpur district of Bangladesh, with the aim of assessing the public health risks associated with these pathogens.
). Pathogenic E. coli and V. cholerae can infect raw vegetables when they are growing in the field, when fertilizer (cow dung) is applied, or when the veggies are harvested, transported, processed, stored, and distributed. Thus far, no reports of outbreaks or sporadic illnesses linked to V. cholerae and E. coli from fresh raw vegetables have come from Bangladesh. In order to isolate E. coli and V. cholerae raw vegetables were gathered near Dinajpur city for this purpose. Therefore, this study was conducted to isolate, identify, and evaluate the microbial loads along with the antimicrobial susceptibility patterns of E. coli and V. cholerae in raw vegetables sold in local markets in the Dinajpur district of Bangladesh, with the aim of assessing the public health risks associated with these pathogens.
2. Materials and Methods
2.1 Ethical approval
No ethical approval is required for this study.
2.2 Study area
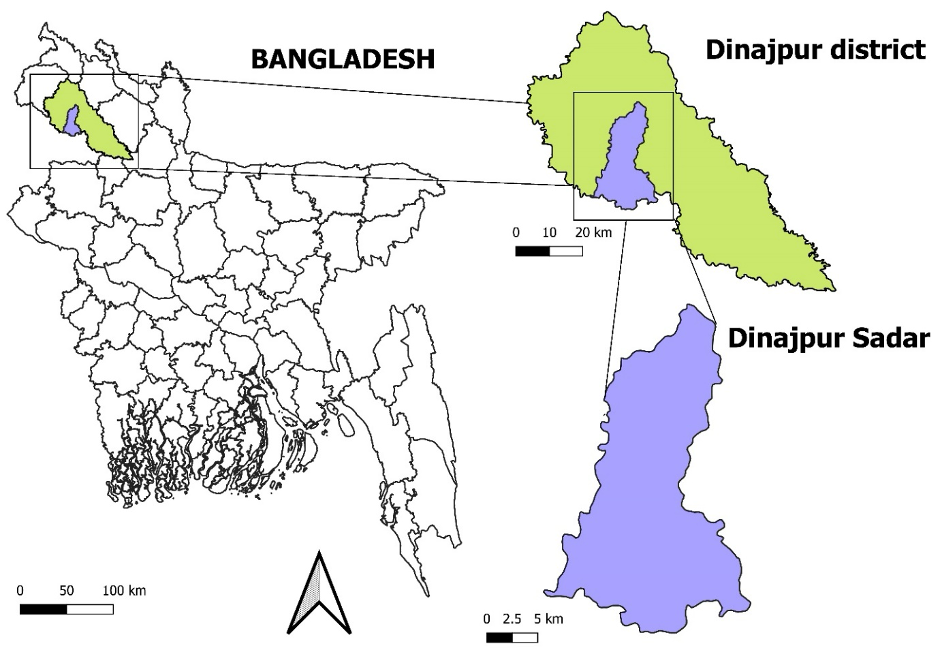
The present study was conducted during the period from December 2019 to March 2020 in the Bacteriology Laboratory of the Department of Microbiology, Hajee Mohammad Danesh Science and Technology University, Dinajpur-5200. The study purposed used samples were collected from 4 different markets (Basher hat, Gopalgonj, Liliar Mor and kalitola) located in the district of Dinajpur Sadar area (Figure 1).
2.3 Sample collection
A total of 35 samples comprising potato (6), cucumber (4), carrot (2), green chili (06), mustard sak (1), cabbage (4), cauliflower (2), coriander leaves (4) , and tomato (6) were collected from 4 different markets (Basher hat, Gopalgonj, Liliar Mor, kalitola) located in the district of Dinajpur area (Figure 1) using hand gloves, sealed poly bags (transparent zip lock poly; thickness: 30-100 mic; size: 175 mm* 100mm). The samples were labeled with an identification mark and quickly moved to the proper containers. The samples were handled with great care, placed in an ice box at 4°C, and then brought right away to bacteriology laboratory, Department of Microbiology, Hajee Mohammad Danesh Science and Technology University, Dinajpur-5200, Bangladesh our lab for examination. The samples are handled with care and stored in a box. Because of aseptic care was taken during transportation and also the samples were kept in sterile container until they're prepared for bacteriological analysis.
2.4 Sample processing
In a sterile mortar and pestle, 10 g of materials were mixed. To create a 10% sample suspension, the samples were then completely homogenized in 90 milliliters of sterile phosphate-buffered saline (PBS; pH 7.4, Merck KGaA, Germany) solution. The sample was diluted ten times, from 10−1 to 10−10, in accordance with the guidelines provided by Qamar et al. (2023 ![]() ). and the International Organization for Standardization (ISO, 1995
). and the International Organization for Standardization (ISO, 1995 ![]() ). Serial dilution was then performed by repeatedly using the previous dilution as the input for the next step, resulting in a reduced number of bacterial colonies to obtain pure colonies. 1 mL of a homogenized sample and 9 mL of sterile PBS were combined in this process. For the isolation of bacteria from vegetables, homogenized samples were incubated at 37°C for 24 hours in order to enrich them in nutrient agar.
). Serial dilution was then performed by repeatedly using the previous dilution as the input for the next step, resulting in a reduced number of bacterial colonies to obtain pure colonies. 1 mL of a homogenized sample and 9 mL of sterile PBS were combined in this process. For the isolation of bacteria from vegetables, homogenized samples were incubated at 37°C for 24 hours in order to enrich them in nutrient agar.
2.5 Total viable count enumeration
The manufacturer's instructions were followed while preparing the nutrient agar media (HiMedia, India), and it was autoclaved for 15 minutes at 121°C to achieve sterilization. Using a micropipette, 0.1 mL of each tenfold dilution was spread out in duplicate onto nutrient agar plates (HiMedia). Using a sterile spreader, the diluted samples were applied to the plate's surface as soon as possible. The plates were incubated for 24 hours at 37°C. The TVC was computed using the ISO methodology (ISO, 1995 ![]() ). Counts were performed on plates showing between 30 and 300 colonies after incubation. The following formula was used to determine CFU/g: number of colonies × dilution factor/volume of culture plated.
). Counts were performed on plates showing between 30 and 300 colonies after incubation. The following formula was used to determine CFU/g: number of colonies × dilution factor/volume of culture plated.
2.6 Isolation and identification of E. coli and V. cholerae
The overnight enriched culture was streaked onto MacConkey (MC) agar, Eosin Methylene Blue (EMB) agar, and Thiosulfate Citrate Bile Salts Sucrose (TCBS) agar (Hi-media), followed by 24 hour incubation at 37°C. MacConkey (MC) agar medium was used for the identification of organism under the family Enterobacteriaceae through studying fermentation characteristics. Eosin methylene blue (EMB) agar medium was used for the purpose of selective growth of E. coli. Thiosulphate citrate bile salt sucrose (TCBS) agar was used for the growth of V. cholerae (Bolinches et al., 1988 ![]() ; Cheesbrough, 1985
; Cheesbrough, 1985 ![]() ).
).
The colonies which produced bright pink colonies on to MacConkey (MC) agar that were selected as E. coli. For the more confirmation of E. coli isolated colonies were sub-cultured onto EMB agar. The colonies that displayed a dark core and a metallic green gloss were chosen. In contrast, the colonies appeared onto yellow and shiny color onto Thiosulphate citrate bile salt sucrose (TCBS) agar that were selected of V. cholerae (Cheesbrough, 2006 ![]() ). The colony characteristics (size, shape, edge, color, and opacity), morphological characteristics by Gram's staining, the sugar fermentation test, and a battery of biochemical tests (methyl red, Voges-Proskauer (VP), catalase, triple sugar iron (TSI) agar slant test, and Simmon's citrate) were used to identify the bacteria (Cheesbrough, 2006
). The colony characteristics (size, shape, edge, color, and opacity), morphological characteristics by Gram's staining, the sugar fermentation test, and a battery of biochemical tests (methyl red, Voges-Proskauer (VP), catalase, triple sugar iron (TSI) agar slant test, and Simmon's citrate) were used to identify the bacteria (Cheesbrough, 2006 ![]() ).
).
2.7 Polymerase chain reaction (PCR)
For the molecular characterization of E. coli and V. cholerae by PCR, the PCR mixture was prepared according to the composition and quantities. The boiling process was utilized to extract deoxyribonucleic acid (DNA) (Aworh et al., 2023 ![]() ). All 16 isolates and 13 isolates, respectively, were subjected to PCR tests for E. coli and V. cholerae confirmation. The highly conserved region of 16S rRNA and groEL were selected for the identification of E. coli and vibrio spp.; the primer list utilized for the PCR experiment is shown in Table 1 (Sarker et al., 2018
). All 16 isolates and 13 isolates, respectively, were subjected to PCR tests for E. coli and V. cholerae confirmation. The highly conserved region of 16S rRNA and groEL were selected for the identification of E. coli and vibrio spp.; the primer list utilized for the PCR experiment is shown in Table 1 (Sarker et al., 2018 ![]() ; Hossain et al., 2014
; Hossain et al., 2014 ![]() ). Multiplex PCR was used to identify the type of bacterium (V. cholerae) (Hossain et al., 2014
). Multiplex PCR was used to identify the type of bacterium (V. cholerae) (Hossain et al., 2014 ![]() ).
).
A 20:1 PCR reaction mixture including 5:1 ribonuclease free water, 10:1 PCR master mixture (Thermo Scientific, EU), 3:1 genomic DNA, and 2:1 primer was used for a single sample. The PCR amplification process involved five minutes of initial denaturation at 95°C, thirty cycles of denaturation at 94°C for forty seconds, primer annealing at 56°C for thirty seconds, and primer extension at 72°C for thirty seconds. For E. coli, the final extension was performed at 72°C for ten minutes. The PCR reaction profile for V. cholerae was also set up with the following temperature parameters: five minutes of initial denaturation at 94°C, thirty cycles of denaturation at 94°C for thirty seconds, primer annealing at 69°C for thirty seconds, and thirty seconds of primer extension at 72°C, culminating in a final extension at 72°C for seven minutes (Sarker et al., 2018 ![]() ).
).
Another for V. cholerae the PCR reaction profile was set with the following thermal conditions: an initial denaturation at 94°C for 5 minutes, followed by 30 cycles of denaturation at 94°C for 30 seconds, primer annealing at 69°C for 30 seconds, and primer extension at 72°C for 30 seconds, with a final extension at 72°C for 7 minutes. Using a primer-specific PCR reaction that targeted the 16S rRNA and groEL gene of E. coli and V. cholerae were molecularly identified (Table 1) (Hossain et al., 2014 ![]() ).
).
Table 1. Primers used for the molecular detection E. coli and Vibrio spp.
2.8 Antibiogram study
Antimicrobial discs with various concentrations were employed to assess the sensitivity of the isolated bacteria. Mueller-Hinton agar (MHA) (HiMedia) was subjected to an antibiotic susceptibility test utilizing the Kirby-Bauer disk diffusion method, as reported by Bauer et al. (1966 ![]() ). The following antibiotic disc was employed for E. coli, ampicillin (30 μg/disc), gentamycin (10 μg/disc), kanamycin (30 μg/disc), colistin (30 μg/disc), nalidixic Acid (30 μg/disc), enrofloxacin (5 μg/disc), oxytetracycline (30 μg/disc), ceftriaxone (30 μg/disc), ceftazidime (30 μg/disc), cefixime (5 μg/disc), and cephalexin (30 μg/disc) (HiMedia).
). The following antibiotic disc was employed for E. coli, ampicillin (30 μg/disc), gentamycin (10 μg/disc), kanamycin (30 μg/disc), colistin (30 μg/disc), nalidixic Acid (30 μg/disc), enrofloxacin (5 μg/disc), oxytetracycline (30 μg/disc), ceftriaxone (30 μg/disc), ceftazidime (30 μg/disc), cefixime (5 μg/disc), and cephalexin (30 μg/disc) (HiMedia).
On the contrast, these antibiotics is applied against V. cholerae gentamycin (10 μg/disc), streptomycin (10 μg/disc), kanamycin (30 μg/disc), trimethoprim (25μg/disc), ciprofloxacin (5 μg/disc), ampicillin (30 μg/disc), nalidixic acid (30 μg/disc), and polymyxin B (5 μg/disc) (HiMedia). The Clinical and Laboratory Standards Institute's guidelines for sensitivity, intermediate, and resistance were compared to the zone sizes of the bacteria (CLSI, 2018 ![]() ).
).
2.9 Statistical analysis
Using Duncan's multiple range test (Statistical Package for the Social Sciences, version 11.5, IBM Corp., NY, USA), the findings of TVC of bacteria detected in the raw vegetables sold at local markets were examined for statistical significance. A statistically significant result was defined as p < 0.05.
3. Results
3.1 Total Viable Count (TVC) of raw vegetable samples
Total Viable Count (TVC) of vegetable samples from markets in Dinajpur district, revealing varying levels of microbial loads. To determine TVC, all thirty-five vegetable samples were tested. The results of the bacteria isolates are shown in Table 2, where the mean values are given in log10 CFU/gm. Based on these findings, the average number of bacteria was calculated from samples of various vegetables and varied from 1.4 ± 0.25 to 2.4 ± 0.37 CFU ± standard deviation (SD)/gm. There were 2.4 ± 0.37 CFU ± SD/gm of bacteria isolated from potato samples, which was the largest number, and 2.4 ± 0.37 CFU ± SD/gm of bacteria recovered from coriander leaves, which was the lowest number (Table 2; Figure 2).
Table 2. Total Viable Count (TVC) of vegetable samples from different markets in Dinajpur district.
3.2 Prevalence of E. coli and V. cholerae
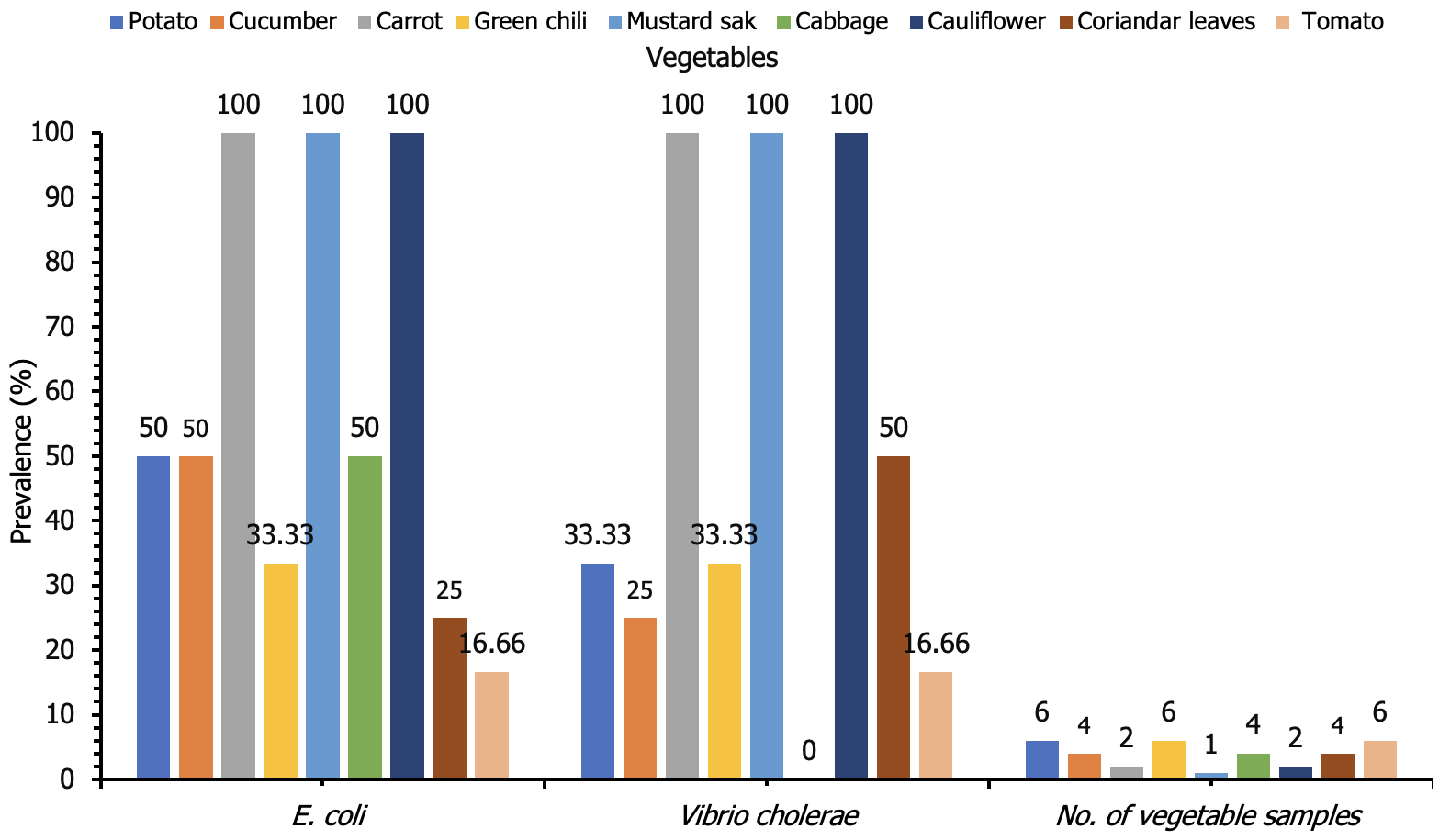
From 35 vegetable samples, 29 distinct bacterial isolates were found in this investigation on the frequency of E. coli and V. cholerae. A total of 16 isolates of E. coli were found in 3 (50%), 2 (50 %), 2 (100%), 2 (33.33%), 1 (100%), 2 (50%), 2 (100%), 1 (25%), and 1 (16.66%) potato, cucumber, carrot, green chili, mustard sak, cabbage, cauliflower, coriander leaves, and tomato vegetables samples (Figure 3). Conversely, 13 isolates of V. cholerae were detected in 2 (33.33%), 1 (25%), 2 (100%), 2 (33.33%), 1 (100%), 0 (0%) 2 (100%), 2 (50%), and 1 (16.66%) potato, cucumber, carrot, green chili, mustard sak, cabbage, cauliflower, coriander leaves, and tomato. Six samples showed no microbial growth. These findings underscore the widespread contamination of raw vegetables by E. coli and V. cholerae, posing potential health risks (Figure 3).
3.3 Cultural, staining, and biochemical characteristics
The cultural characteristics of bacterial isolates obtained from vegetable samples. E. coli colonies on MC agar exhibited a distinctive bright pink color colonies and EMB agar showed a blue-green metallic sheen color colonies. In contrast, V. cholerae colonies on TCBS agar appeared yellow and shiny (Figure 4 and 5). Gram staining showed that E. coli produces short, pink, rod-shaped, single or paired Gram-negative bacteria. On the other hand, gram staining V. cholerae expressed gram negative, curved rod, single or paired and motile. Most of the isolates of E. coli showed positive results for methyl red (MR), indole, and TSI (with yellow on butt and slant, and positive for H2S production), but negative for Voges-Proskauer (VP) and citrate utilization (CT). Similarly most of the isolates V. cholerae, exhibited positive results for MR, indole, TSI (yellow on butt and slant), and citrate utilization, while showing negative results for VP and gas production in TSI.
3.4 Molecular detection
3.4.1 Molecular confirmation of E. coli targeting 16S rRNA gene by PCR
All 16 culture positive isolates of E. coli were tested for PCR targeting the 16S rRNA gene for amplification of 704 bp of DNA fragment and 16 of 5 (31.25%) isolates showed positive result in PCR that isolated from vegetable (Table 3 and Figure 6).



3.4.2 Molecular confirmation of Vibrio spp. and V. cholerae targeting groEL gene and multiplex PCR
Among the 13 positive suspected Vibrio isolates, 4 (30.76%) were confirmed as Vibrio spp. when genus specific groEL primers were used and a positive band appeared at 1117 bp (Table 3 and Figure 7). All the four Vibrio spp. were confirmed as V. cholerae when multiplex PCR was performed using species specific groEL primers and positive band was appeared at 418 bp (Figure 8). No isolate was detected as V. parahaemolyticus, V. alginolyticus and V. vulnificus by multiplex PCR.

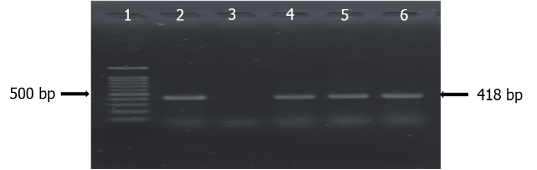
Table 3. Overall Molecular prevalence of 16S rRNA gene of E. coli and groEL gene of V. cholerae isolated from raw vegetables.
3.5 Antibiogram profiles
E. coli and V. cholerae isolates tested positive for antibiotics, showing that they were resistant, intermediate and susceptible. Antibiotic susceptibility tests were performed on all 16 and 13 culture-positive isolates of E. coli and V. cholerae, respectively, using 11 and 8 different antibiotics under 7 and 5 distinct antibiotic classes (Figure 9 and 10).
All E. coli isolates (n=16) showed various resistance pattern against of all antibiotics. But E. coli isolates were highly resistant to 93.75% against ceftazidime, 87.25% against cefixime, 81.25% against ampicillin, 75% against oxytetracycline, respectively. Besides, highly sensitive isolates were exhibited 87.50%, 81.25%, and 68.75% against gentamycin, ceftriaxone, colistin, and enrofloxacin (Figure 9).
Another, 13 V. cholerae isolates 92.30% resistant to nalidixic acid, 76.92% against trimethoprim, 69.23% against polymyxin, 53.84% against ciprofloxacin and amoxicillin. Conversely, highly sensitive isolates were exhibited 92.30%, 84.61%, and 53.84% against kanamycin, gentamicin, and streptomycin (Figure 10).
3.6 MDR profile of E. coli and V. cholerae isolated from vegetable sample
Out of 16 culture positive E. coli isolates, 10 (62.50%) were MDR in terms of phenotype. A total of 11 resistance patterns were observed where six were MDR patterns. The MDR prevalence was 62.50%. The MDR pattern numbers 4 (AMP-OTC-CAZ-CFX-SEF), 2 (AMP-K-OTC-CAZ-CFX-SEF), and 2 (OTC-CAZ-CFX-SEF) were observed in the highest number of MDR E. coli isolates. The resistance pattern number 2 (OTC-CAZ-CFX-SEF), 1 (AMP-CAZ-CFX-SEF), 1 (AMP-CAZ-CFX), 1 (AMP-CAZ-CFX), and 1 (AMP-CAZ) was not phenotypically MDR in nature. Every E. coli isolate had a different profile of antibiotic resistance, with MAR indices ranging from 0.27 to 0.72 (Table 4).
Other 13 culture positive V. cholerae isolates, 12 (92.30%) were phenotypically MDR in nature. A total of 10 resistance patterns were observed where 9 were MDR patterns. The MDR prevalence was 92.30%. The MDR pattern numbers 2 (W-CIP-AMP-NA-PB), 2 (W-AMP-NA-PB), and 2 (W-CIP-NA) were observed in the highest number of MDR V. cholerae isolates. The resistance pattern number 1 (W-PB) was not phenotypically MDR in nature. Each V. cholerae isolate's profile of antibiotic resistance was found to be unique, with MAR indices ranging from 0.25 to 1. (Table 5).
4. Discussion
Fresh vegetables are widely recognized for their nutritional benefits, providing essential vitamins, minerals, and fiber crucial for maintaining good health. However, concerns about potential health hazards associated with their production and consumption persist. A significant issue arises from the use of chemical fertilizers in vegetable farming, which can introduce harmful toxins into the produce, thereby posing risks to consumers (Mostafidi et al., 2020 ![]() ). Additionally, several multidrug resistance isolates associated with fresh vegetables which are responsible for different infectious diseases in human health worldwide. Customers are more concerned with maintaining good eating habits and their health as they are becoming more aware of the health benefits of fresh fruits and vegetables. However, concurrently with the rise in fresh produce-related foodborne illness outbreak (Nagyová et al., 2019
). Additionally, several multidrug resistance isolates associated with fresh vegetables which are responsible for different infectious diseases in human health worldwide. Customers are more concerned with maintaining good eating habits and their health as they are becoming more aware of the health benefits of fresh fruits and vegetables. However, concurrently with the rise in fresh produce-related foodborne illness outbreak (Nagyová et al., 2019 ![]() ).
).

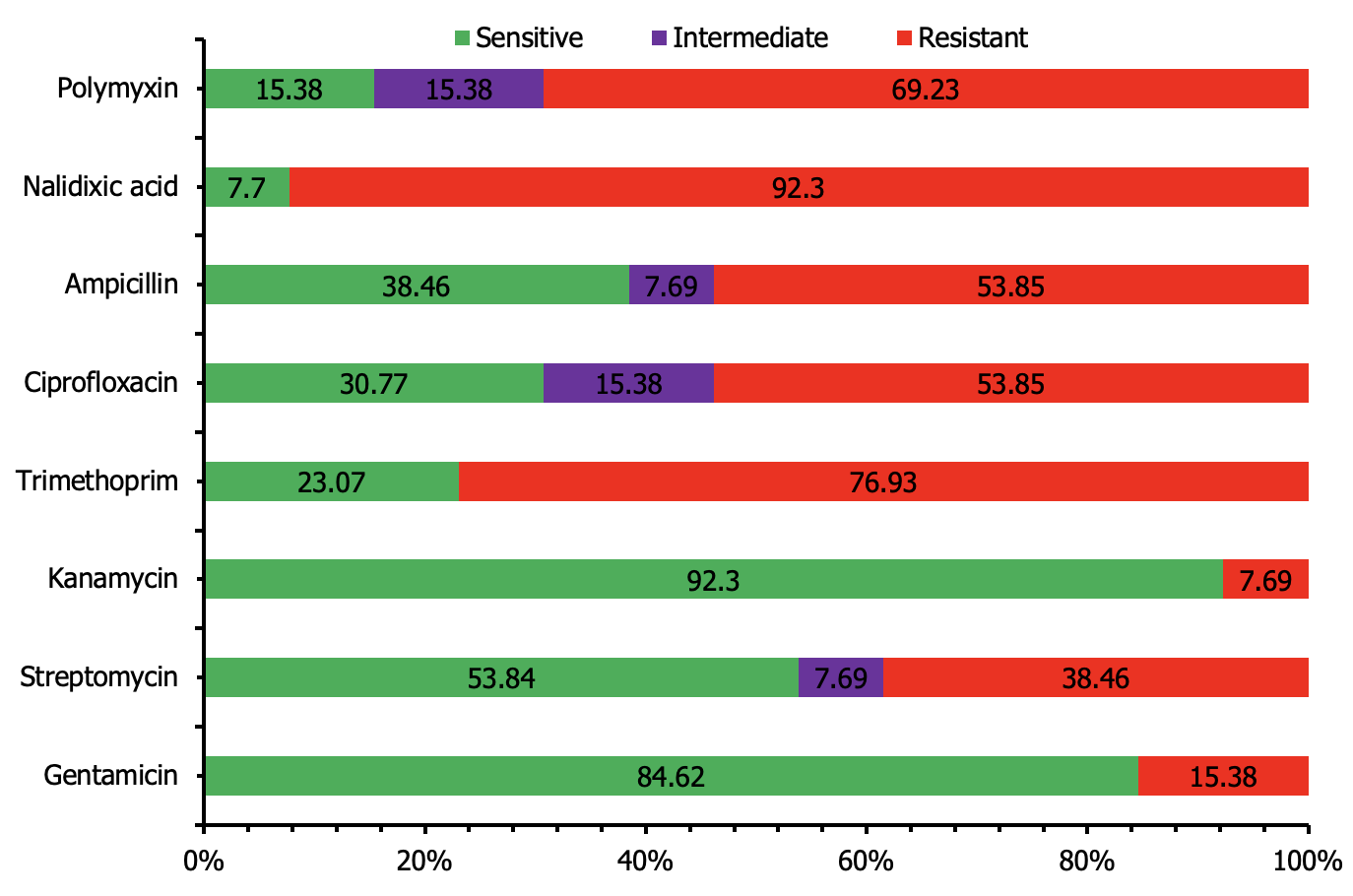
Table 4. Multidrug resistant profile of E. coli isolated from raw vegetable samples.
Table 5. Multidrug resistant profile of V. cholerae isolated from raw vegetable samples.
Recent research has highlighted the presence of multidrug-resistant bacteria such as E. coli and V. cholerae in fresh vegetables, posing global public health challenges due to their potential to cause infectious diseases (Sultana et al., 2021 ![]() ). The study conducted in northern Bangladesh have specifically identified these pathogens in vegetable samples, revealing patterns of resistance to antibiotics commonly used in clinical settings. In this molecular study, we isolated two most important multidrug resistance isolates including E. coli and V. cholerae from different vegetables samples.
). The study conducted in northern Bangladesh have specifically identified these pathogens in vegetable samples, revealing patterns of resistance to antibiotics commonly used in clinical settings. In this molecular study, we isolated two most important multidrug resistance isolates including E. coli and V. cholerae from different vegetables samples.
Many research have looked into the possible pre and post-harvest (in the field) and post-harvest causes of product contamination within the supply chain. Pathogen communities have the ability to establish themselves on developing crops throughout the pre-harvest stage. After harvest, the likelihood will increase due to either further direct contamination or the expansion of already-existing pathogen populations during processing and handling methods. In the field, water will most likely be a major source of contamination. Runoff from neighboring animal pastures and irrigation from a contaminated source are potential contributors (Iwu and Okoh, 2019 ![]() ). The risk associated with using water from resources that change in microbiological quality for irrigation is the cause of surface and ground water contamination in Bangladesh, indicating the need for stronger regulations (Bilal et al., 2023
). The risk associated with using water from resources that change in microbiological quality for irrigation is the cause of surface and ground water contamination in Bangladesh, indicating the need for stronger regulations (Bilal et al., 2023 ![]() ; Jeong et al., 2016
; Jeong et al., 2016 ![]() ; Uyttendaele et al., 2015
; Uyttendaele et al., 2015 ![]() ). There are several documented pathways of water contamination than irrigation. One potential source of contamination is insects. Contaminated et al flies have been demonstrated to directly transfer bacteria to plant leaves or fruits in scientific settings (Yin et al., 2022
). There are several documented pathways of water contamination than irrigation. One potential source of contamination is insects. Contaminated et al flies have been demonstrated to directly transfer bacteria to plant leaves or fruits in scientific settings (Yin et al., 2022 ![]() ; Osafo et al., 2022
; Osafo et al., 2022 ![]() ; Wasala et al., 2013
; Wasala et al., 2013 ![]() ).
).
The Total Viable Count (TVC) analysis of vegetable samples from markets in Dinajpur district revealed significant variations in bacterial contamination levels. The results indicated an average bacterial count ranging from 1.4 ± 0.25 to 2.4 ± 0.37 CFU ± standard deviation (SD)/g, with potatoes showing the highest contamination (2.4 ± 0.37 CFU ± SD/g) and coriander leaves the lowest (2.4 ± 0.37 CFU ± SD/g). These findings are critical in understanding the microbial safety of fresh produce in the region. Aurin et al. (2020 ![]() ) observed a similar spectrum of microbial loads in fresh vegetables from Dhaka markets, with TVC ranging from 8×107 to 1.70×108 CFU/g. Further, Mahfuza et al. (2016
) observed a similar spectrum of microbial loads in fresh vegetables from Dhaka markets, with TVC ranging from 8×107 to 1.70×108 CFU/g. Further, Mahfuza et al. (2016 ![]() ) and Sultana et al. (2021
) and Sultana et al. (2021 ![]() ) reported substantial ranges in TVC (8×103 to 2.1×108 CFU/ml) and total coliform counts (TCC) (1.5×104 to 2.2×108 CFU/ml) in various fruits, salad vegetables and juices different areas in Dhaka city, Bangladesh. Similarly, Mrityunjoy et al. (2013
) reported substantial ranges in TVC (8×103 to 2.1×108 CFU/ml) and total coliform counts (TCC) (1.5×104 to 2.2×108 CFU/ml) in various fruits, salad vegetables and juices different areas in Dhaka city, Bangladesh. Similarly, Mrityunjoy et al. (2013 ![]() ) reported that the V. cholerae was found 5.1×106, 4.1×105, 4.2×105, 1.1×107, 7.6×106, 2.5×105, and 8.25×105 cfu/g or cfu/ml in cucumber, carrot, tomoto, cauliflower, cabbage, kolmisak, and helenchasak in the Malibag market of Dhaka city, Bangladesh. These studies collectively underscore the widespread nature of microbial contamination, reflecting ongoing challenges in maintaining food safety standards. The quality of irrigation water, a significant factor, can introduce pathogens from animal waste and environmental runoff (Jeong et al., 2016
) reported that the V. cholerae was found 5.1×106, 4.1×105, 4.2×105, 1.1×107, 7.6×106, 2.5×105, and 8.25×105 cfu/g or cfu/ml in cucumber, carrot, tomoto, cauliflower, cabbage, kolmisak, and helenchasak in the Malibag market of Dhaka city, Bangladesh. These studies collectively underscore the widespread nature of microbial contamination, reflecting ongoing challenges in maintaining food safety standards. The quality of irrigation water, a significant factor, can introduce pathogens from animal waste and environmental runoff (Jeong et al., 2016 ![]() ; Uyttendaele et al., 2015
; Uyttendaele et al., 2015 ![]() ). Insects, particularly flies, have also been identified as vectors that can transfer bacteria directly to vegetable surfaces during growth and handling (Osafo et al., 2022
). Insects, particularly flies, have also been identified as vectors that can transfer bacteria directly to vegetable surfaces during growth and handling (Osafo et al., 2022 ![]() ; Wasala et al., 2013
; Wasala et al., 2013 ![]() ). These vectors contribute to the contamination observed across different studies and regions.
). These vectors contribute to the contamination observed across different studies and regions.
In our study, we found that E. coli exhibited the highest prevalence, with 35 of 16 (45.71%) strains detected accounting for of the tested samples. and V. cholera was also notably present, with 35 of 13 (37.14%) strains identified, making up the samples analyzed. The prevalence of E. coli in our study was higher than the finding of Ratshilingano et al. (2022 ![]() ), who observed out of 51 E. coli isolates, including 28 E. coli were obtained from 17.4% (11 of 63) of the water samples, 11.8% (16 of 136) leafy green samples. In our study, the prevalence of V. cholerae was lower than the findings of Sultana et al. (2021
), who observed out of 51 E. coli isolates, including 28 E. coli were obtained from 17.4% (11 of 63) of the water samples, 11.8% (16 of 136) leafy green samples. In our study, the prevalence of V. cholerae was lower than the findings of Sultana et al. (2021 ![]() ), who conducted a study where out of 20, 8 (40%) isolates were found to be positive for V. cholerae in street fruits and juices from different areas in Dhaka City, Bangladesh. These findings underscore the significant presence of these pathogenic bacteria in fresh vegetable samples, highlighting potential risks associated with their consumption if not properly handled and cooked.
), who conducted a study where out of 20, 8 (40%) isolates were found to be positive for V. cholerae in street fruits and juices from different areas in Dhaka City, Bangladesh. These findings underscore the significant presence of these pathogenic bacteria in fresh vegetable samples, highlighting potential risks associated with their consumption if not properly handled and cooked.
Out of sixteen culture positive E. coli, 16S rRNA gene was confirmed to be present in of 5 (31.25%) isolates in our study. In another study, the similar report supported by Datta et al. (2024 ![]() ) reported that the prevalence of E. coli was found 32.0% (96/300) in selected vegetables and herbs in Bangkok, Thailand. Besides, in our research, among the thirteen culture positive suspected Vibrio isolates, 4 (30.76%) were confirmed as molecular-positive Vibrio spp. All the four Vibrio spp. were confirmed as V. cholera through multiplex PCR. The molecular prevalence of V. cholerae in our study supported by Rivera et al. (2001
) reported that the prevalence of E. coli was found 32.0% (96/300) in selected vegetables and herbs in Bangkok, Thailand. Besides, in our research, among the thirteen culture positive suspected Vibrio isolates, 4 (30.76%) were confirmed as molecular-positive Vibrio spp. All the four Vibrio spp. were confirmed as V. cholera through multiplex PCR. The molecular prevalence of V. cholerae in our study supported by Rivera et al. (2001 ![]() ), who observed that out of 40 suspected Vibrio isolates, 12 (30%) were confirmed as Vibrio spp. using genus-specific groEL primers and further multiplex PCR analysis identified 8 of these 12 isolates as Vibrio cholerae. On the contrary, the prevalence of groEL gene-positive V. cholerae was higher than that of Singh et al. (2002
), who observed that out of 40 suspected Vibrio isolates, 12 (30%) were confirmed as Vibrio spp. using genus-specific groEL primers and further multiplex PCR analysis identified 8 of these 12 isolates as Vibrio cholerae. On the contrary, the prevalence of groEL gene-positive V. cholerae was higher than that of Singh et al. (2002 ![]() ), who revealed that out of 50 samples, 10 (20%) were suspected to be Vibrio spp. through initial screening. When tested with groEL primers, 3 (30%) of these suspected isolates were confirmed as Vibrio spp. by the appearance of a positive band at 1117 bp. Further analysis using species-specific primers confirmed all 3 isolates as Vibrio cholerae, showing a positive band at 418 bp.
), who revealed that out of 50 samples, 10 (20%) were suspected to be Vibrio spp. through initial screening. When tested with groEL primers, 3 (30%) of these suspected isolates were confirmed as Vibrio spp. by the appearance of a positive band at 1117 bp. Further analysis using species-specific primers confirmed all 3 isolates as Vibrio cholerae, showing a positive band at 418 bp.
In our study, E. coli isolated from vegetable samples exhibited high resistance rates to ceftazidime (93.75%), cefixime (87.25%), and ampicillin (81.25%), while demonstrating relatively higher sensitivity to gentamycin (87.50%), ceftriaxone (81.25%), and enrofloxacin (68.75%). In contrast, findings from another study by Aurin et al. (2020 ![]() ) showed the comparable resistance trends but with different antibiotics who reported high resistance rates among E. coli strains to ceftriaxone (100%), nitrofurantoin (94%), erythromycin (89%), and amoxicillin (83%) had the highest resistance against the isolated organisms along with Imipenem showed the highest sensitivity (86%) in fresh leafy and salad vegetable samples. The other study conducted by Alabi et al. (2022
) showed the comparable resistance trends but with different antibiotics who reported high resistance rates among E. coli strains to ceftriaxone (100%), nitrofurantoin (94%), erythromycin (89%), and amoxicillin (83%) had the highest resistance against the isolated organisms along with Imipenem showed the highest sensitivity (86%) in fresh leafy and salad vegetable samples. The other study conducted by Alabi et al. (2022 ![]() ) found that the most susceptible antibiotics to E. coli isolated from water leaves were imipenem (83.3%), then gentamicin (77.8%), ciprofloxacin (66.7%), ceftazidime (61.1%), ampicillin (55.6%), amoxicillin-clavulanic acid (50.0%), cefotaxime (44.4%), cefoxitin (33.3%), septrin (38.8%), and streptomycin (25.9%). Another study coducted by Habib et al. (2023
) found that the most susceptible antibiotics to E. coli isolated from water leaves were imipenem (83.3%), then gentamicin (77.8%), ciprofloxacin (66.7%), ceftazidime (61.1%), ampicillin (55.6%), amoxicillin-clavulanic acid (50.0%), cefotaxime (44.4%), cefoxitin (33.3%), septrin (38.8%), and streptomycin (25.9%). Another study coducted by Habib et al. (2023 ![]() ), who examined the E. coli (n=145) was isolated from fresh salad vegetables, the isolates with the highest phenotypic resistance to ampicillin (20.68%), tetracycline (20%), and trimethoprim-sulfamethoxazole (10.35%) were investigated for antimicrobial resistance.
), who examined the E. coli (n=145) was isolated from fresh salad vegetables, the isolates with the highest phenotypic resistance to ampicillin (20.68%), tetracycline (20%), and trimethoprim-sulfamethoxazole (10.35%) were investigated for antimicrobial resistance.
We isolated 13 Vibrio cholerae isolates where 92.30% resistant to nalidixic acid, 76.92% against trimethoprim, 69.23% against polymyxin, 53.84% against ciprofloxacin and amoxicillin. Conversely, highly sensitive isolates were exhibited 92.30%, 84.61%, and 53.84% against kanamycin, gentamicin, and streptomycin. The another finding conducted by Dalsgaard et al. (2000 ![]() ) reported that isolates of all Vibrio cholerae from raw vegetable samples found that 90% of the isolates were resistant to nalidixic acid, 80% to trimethoprim, and 70% to polymyxin B. Resistance to ciprofloxacin and amoxicillin was observed in 60% and 50% of the isolates, respectively. In contrast, high sensitivity was observed against kanamycin (95%), gentamicin (85%), and streptomycin (60%). Budiman et al. (2022
) reported that isolates of all Vibrio cholerae from raw vegetable samples found that 90% of the isolates were resistant to nalidixic acid, 80% to trimethoprim, and 70% to polymyxin B. Resistance to ciprofloxacin and amoxicillin was observed in 60% and 50% of the isolates, respectively. In contrast, high sensitivity was observed against kanamycin (95%), gentamicin (85%), and streptomycin (60%). Budiman et al. (2022 ![]() ) reported that the antibiotic resistance analysis showed 4.35% isolates of V. cholerae were resistant to gentamicin, streptomycin (17.39%), trimethoprim (52.17%), ciprofloxacin (30.43%), ampicillin (13.04%), nalidixic acid (82.61%), and polymyxin B (91.30%). This highlights the significant challenge of antibiotic resistance in foodborne pathogens and underscores the need for effective surveillance and intervention strategies.
) reported that the antibiotic resistance analysis showed 4.35% isolates of V. cholerae were resistant to gentamicin, streptomycin (17.39%), trimethoprim (52.17%), ciprofloxacin (30.43%), ampicillin (13.04%), nalidixic acid (82.61%), and polymyxin B (91.30%). This highlights the significant challenge of antibiotic resistance in foodborne pathogens and underscores the need for effective surveillance and intervention strategies.
The findings from this study reveal significant concerns regarding multidrug-resistant (MDR) E. coli and V. cholerae isolates in vegetable samples. Out of the 16 culture-positive E. coli isolates, 10 (62.50%) exhibited MDR phenotypes, with a total of 11 resistance patterns observed, including 6 MDR patterns. The prevalence of MDR among E. coli isolates was notably high at 62.50%, with patterns such as AMP-OTC-CAZ-CFX-SEF, AMP-K-OTC-CAZ-CFX-SEF, and OTC-CAZ-CFX-SEF being most frequent.
In contrast, all 13 culture-positive V. cholerae isolates were phenotypically MDR, accounting for 92.30% prevalence across 10 observed resistance patterns, 9 of which were MDR. Common MDR patterns included W-CIP-AMP-NA-PB, W-AMP-NA-PB, and W-CIP-NA. Another study noted significant antimicrobial resistance in foodborne pathogens including E. coli and V. cholerae, with moderate levels of multi drug resistance patterns included common antibiotics like ciprofloxacin and ampicillin (Yu et al., 2019 ![]() ). Notably, each isolate of E. coli and V. cholerae exhibited a unique antibiotic resistance profile, with multiple antibiotic resistance (MAR) indices ranging from 0.25 to 1. The presence of high percentage MDR bacteria in raw vegetables in the northern district of Bangladesh compared to others study, possibly due to differences in local agricultural practices or environmental factors. These findings underscore the serious health risks associated with the presence of MDR E. coli and V. cholerae in vegetable samples, highlighting the urgent need for enhanced surveillance and mitigation strategies to ensure food safety and public health.
). Notably, each isolate of E. coli and V. cholerae exhibited a unique antibiotic resistance profile, with multiple antibiotic resistance (MAR) indices ranging from 0.25 to 1. The presence of high percentage MDR bacteria in raw vegetables in the northern district of Bangladesh compared to others study, possibly due to differences in local agricultural practices or environmental factors. These findings underscore the serious health risks associated with the presence of MDR E. coli and V. cholerae in vegetable samples, highlighting the urgent need for enhanced surveillance and mitigation strategies to ensure food safety and public health.
Both studies highlight the urgent need for enhanced surveillance and control measures to mitigate the spread of antibiotic-resistant bacteria through vegetables. The similarities in resistance profiles across studies indicate a consistent challenge in managing bacterial contamination in agricultural settings, emphasizing the importance of rigorous food safety protocols and antibiotic stewardship to protect public health.
Given these findings, it is vital to follow food safety practices when handling and consuming fresh vegetables. Proper washing and boiling of vegetables are critical steps in reducing the danger of bacterial illnesses. Furthermore, there is an urgent need for proper agricultural laws and monitoring to protect public health against microbial contamination in fresh produce (Nagyová et al., 2019 ![]() ). This study emphasizes the necessity of ongoing efforts to improve food safety procedures and raise consumer awareness about the safe handling and eating of fresh vegetables worldwide.
). This study emphasizes the necessity of ongoing efforts to improve food safety procedures and raise consumer awareness about the safe handling and eating of fresh vegetables worldwide.
5. Conclusions
The current study provided thorough information about the prevalence, characterization, and antibiotic resistance profiles of Escherichia coli and V. cholerae isolated from raw vegetables in Dinajpur district, Bangladesh. The study revealed the presence of MDR strains, which demonstrated considerable resistance to important antibiotics such as ampicillin, ceftazidime, and nalidixic acid. These findings highlight the critical public health concern posed by MDR bacteria in raw vegetables, indicating potential health concerns associated with their use. Addressing these difficulties would necessitate coordinated efforts to implement strict food safety rules, promote healthy agricultural practices, and raise awareness among producers and consumers alike. Furthermore, constant monitoring of antibiotic usage in agricultural and clinical contexts is critical to minimize the rise of antimicrobial resistance pattern.
Acknowledgements
The authors like to thank the Department of Microbiology, Hajee Mohammed Danesh Science and Technology, Dinajpur, Bangladesh for providing necessary supports for conducting the research.
Data availability statement
The data generated from this study will be available on the valid request from the corresponding author.
Informed consent statement
No informed consent was required to conduct the study.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Conceptualization: Anwar Hossain Rana, Md. Khaled Hossain, and Farzana Afroz; Data collection, study conduct, and manuscript write up: Anwar Hossain Rana; Methodology preparation, manuscript write up, molecular data analysis, formatting, and reviewed the final version manuscript: Palash Bose; Data analysis, first draft develop, and result section interpretation: Kazi Abdus Sobur, Sakib Mowdood, and Md. Mosharraf Hossen; Figure preparation and formal analysis: Nazmi Ara Rumi, Mahmudul Hasan, and Nusrat Jahan. Reviewed and revised the final version: Atikur Rahman Titas, Palash Bose, and Md. Aoulad Hosen. All authors critically reviewed the manuscript and agreed to submit final version of the manuscript.
Reference
(Silva Dias, 2022)
Silva Dias JC, 2022. Nutritional quality and health benefits of vegetables. Emerging Trends in Disease and Health Research, 4: 7–35. https://doi.org/10.9734/bpi/etdhr/v4/15660Dv
Reference
(Rudzińska et al., 2023).
Rudzińska A, Juchaniuk P, Oberda J, Wiśniewska J, Wojdan W, Szklener K, Mańdziuk S, 2023. Phytochemicals in cancer treatment and cancer prevention—review on epidemiological data and clinical trials. Nutrients, 15(8): 1896. https://doi.org/10.3390/nu15081896
Reference
(Pinto et al., 2021)
Pinto T, Aires A, Cosme F, Bacelar E, Morais MC, Oliveira I, Gonçalves B, 2021. Bioactive (poly) phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods, 10: 106. https://doi.org/10.3390/foods10010106
Reference
(Sotler et al., 2019)
Sotler R, Poljšak B, Dahmane R, Jukić T, Pavan Jukić D, Rotim C and Starc A 2019. Prooxidant activities of antioxidants and their impact on health. Acta Clinica Croatica, 58(4): 726-736. https://doi.org/10.20471/acc.2019.58.04.20
Reference
(Dias, 2012).
Dias, 2012. Nutritional quality and health benefits of vegetables: A review. Food and Nutrition Sciences, 3(10): 1354-1374. https://doi.org/10.4236/fns.2012.310179
Reference
(Haque and Hoque, 2021)
Haque MM and Hoque MZ, 2021. Vegetable production and marketing channels in Bangladesh: Present scenario, problems, and prospects. In Seminar Paper.
Reference
(Aalm and Nase, 2020)
Alam SN and Naser MN, 2020. Nutritional and health issues in Bangladesh and solutions through traditional foods. In: Nutritional and Health Aspects of Food in South Asian Countries (pp. 237-254). Academic Press. https://doi.org/10.1016/B978-0-12-820011-7.00026-5
Reference
(Bekele et al., 2017)
Bekele F, Tefera T, Biresaw G and Yohannes T, 2017. Parasitic contamination of raw vegetables and fruits collected from selected local markets in Arba Minch town, Southern Ethiopia. Infectious Diseases of Poverty, 6: 1-7. https://doi.org/10.1186/s40249-016-0226-6
Reference
(Desiree et al., 2021)
Desiree K, Schwan CL, Ly V, Hok L, Bello NM, Nwadike L and Vipham JL, 2021. Investigating Salmonella enterica, Escherichia coli, and coliforms on fresh vegetables sold in informal markets in Cambodia. Journal of Food Protection, 84(5): 843-849. https://doi.org/10.4315/JFP-20-219
Reference
(Said, 2012)
Said DES, 2012. Detection of parasites in commonly consumed raw vegetables. Alexandria Journal of Medicine, 48(4): 345-352. https://doi.org/10.1016/j.ajme.2012.05.005
Reference
(Alegbeleye et al., 2023).
Alegbeleye O and Sant’Ana AS, 2023. Microbiological quality of irrigation water for cultivation of fruits and vegetables: An overview of available guidelines, water testing strategies and some factors that influence compliance. Environmental Research, 220: 114771. https://doi.org/10.1016/j.envres.2022.114771
Reference
(Łepecka et al., 2022).
Łepecka A, Zielińska DS, zymański P, Buras I and Kołożyn-Krajewska D, 2022. Assessment of the microbiological quality of ready-to-eat salads—are there any reasons for concern about public health? International Journal of Environmental Research and Public Health, 19(3): 1582. https://doi.org/10.3390/ijerph19031582
Reference
(Osafo et al., 2022).
Osafo R, Balali GI, Amissah-Reynolds PK, Gyapong F, Addy R, Nyarko AA and Wiafe P, 2022. Microbial and parasitic contamination of vegetables in developing countries and their food safety guidelines. Journal of Food Quality, 2022: 4141914. https://doi.org/10.1155/2022/4141914
Reference
(Machado‐Moreira et al., 2019).
Machado‐Moreira B, Richards K, Brennan F, Abram F and Burgess CM, 2019. Microbial contamination of fresh produce: what, where, and how? Comprehensive reviews in food science and food safety, 18(6): 1727-1750. https://doi.org/10.1111/1541-4337.12487
Reference
(Alegbeleye et al., 2022).
Alegbeleye O, Odeyemi OA, Strateva M and Stratev D, 2022. Microbial spoilage of vegetables, fruits and cereals. Applied Food Research, 2: 100122. https://doi.org/10.1016/j.afres.2022.100122
Reference
(Islam et al., 2023).
Islam S, Thangadurai D, Sangeetha J and Cruz-Martins N, 2023. Global food safety: Microbial interventions and molecular advancements. CRC Press. pp. 366.
Reference
(Julqarnain et al., 2022).
Julqarnain SM, Bose P, Rahman MZ, Khatun MM and Islam MA, 2022. Bacteriological quality and prevalence of foodborne bacteria in broiler meat sold at live bird markets at Mymensingh City in Bangladesh. Journal of Advanced Veterinary and Animal Research, 9(3): 405. http://doi.org/10.5455/javar.2022.i608
Reference
(Hossain et al., 2024)
Hossain MS, Jony MAH, Saha N, Islam B, Sobur KA, Mowdood S and Hossain KM, 2024. Antibiotic Resistance Genes Detection in Escherichia coli Isolated from Raw Meat in Rajshahi Division of Bangladesh. American Journal of Microbiological Research, 12(4): 79-84. https://doi.org/10.12691/ajmr-12-4-1
Reference
(Ema et al., 2022)
Ema FA, Shanta RN, Rahman MZ, Islam MA and Khatun MM, 2022. Isolation, identification, and antibiogram studies of Escherichia coli from ready-to-eat foods in Mymensingh, Bangladesh. Veterinary World, 15(6): 1497. http://doi.org/10.14202/vetworld.2022.1497-1505
Reference
(Ongeng et al., 2015)
Ongeng D, Geeraerd AH, Springael D, Ryckeboer J, Muyanja C and Mauriello G, 2015. Fate of Escherichia coli O157: H7 and Salmonella enterica in the manure-amended soil-plant ecosystem of fresh vegetable crops: A review. Critical reviews in microbiology, 41(3): 273-294. https://doi.org/10.3109/1040841X.2013.829415
Reference
(Weil and Harris, 2015)
Weil AA and Harris JB, 2015. V. cholerae. In: Molecular medical microbiology (pp. 1079-1098). Academic Press. https://doi.org/10.1016/B978-0-12-397169-2.00060-3
Reference
(Abioye et al., 2021)
Abioye OE, Osunla AC and Okoh AI, 2021. Molecular detection and distribution of six medically important Vibrio spp. in selected freshwater and brackish water resources in Eastern Cape Province, South Africa. Frontiers in Microbiology, 12: 617703. https://doi.org/10.3389/fmicb.2021.617703
Reference
(Chowdhury et al., 2022)
Chowdhury F, Ross AG, Islam MT, McMillan NA and Qadri F, 2022. Diagnosis, management, and future control of cholera. Clinical Microbiology Reviews, 35(3): e00211-21. https://doi.org/10.1128/cmr.00211-21
Reference
(Baker-Austin et al., 2018)
Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J, 2018. Vibrio spp. infections. Nature Reviews Disease Primers, 4: 1-19. https://doi.org/10.1038/s41572-018-0005-8
Reference
(Rebaudet et al., 2013)
Rebaudet S, Sudre B, Faucher B and Piarroux R, 2013. Environmental determinants of cholera outbreaks in inland Africa: a systematic review of main transmission foci and propagation routes. The Journal of Infectious Diseases, 208: S46-S54. https://doi.org/10.1093/infdis/jit195
Reference
(Fleckenstein and Kuhlmann, 2019)
Fleckenstein JM and Kuhlmann FM, 2019. Enterotoxigenic Escherichia coli infections. Current infectious disease reports, 21: 1-9. https://doi.org/10.1007/s11908-019-0665-x
Reference
(Aurin et al., 2020)
Aurin SA, Chowdhury SP, Abony M, Rifa J, Banik A, Fatema AN and Ahmed Z, 2020. Characterization of multi-drug resistant gram-negative bacteria present in fresh leafy and salad vegetables in Dhaka, Bangladesh. European Journal of Engineering and Technology Research, 5(11): 1322-1327. https://doi.org/10.24018/ejers.2020.5.11.2212
Reference
Qamar et al. (2023)
Qamar MU, Rizwan M, Uppal R, Khan AA, Saeed U, Ahmad K and Suleman M, 2023. Antimicrobial susceptibility and clinical characteristics of multidrug-resistant polymicrobial infections in Pakistan, a retrospective study 2019–2021. Future Microbiology, 18(17): 1265-1277. https://doi.org/10.2217/fmb-2023-0110
Reference
(ISO, 1995)
ISO, 1995. Recommendation of the meeting of the subcommittee, international organization for standardization on meat and meat products. ISO/TC-36/ SC 6.10-18. ISO, Geneva, Switzerland.
Reference
(Bolinches et al., 1988)
Bolinches J, Romalde JL and Toranzo AE, 1988. Evaluation of selective media for isolation and enumeration of vibrios from estuarine waters. Journal of microbiological methods, 8(3): 151-160. https://doi.org/10.1016/0167-7012(88)90016-4
Reference
(Cheesbrough, 1985)
Cheesbrough M, 1985. Medical laboratory manual for tropical countries. English Language Book Society, London. pp. 479.
Reference
(Cheesbrough, 2006)
Cheesbrough M, 2006. District laboratory practice in tropical countries. Cambridge University Press, Cambridge, UK. pp. 434.
Reference
(Aworh et al., 2023)
Aworh MK, Kwaga JK, Hendriksen RS, Okolocha EC, Harrell E and Thakur S, 2023. Quinolone-resistant Escherichia coli at the interface between humans, poultry and their shared environment-a potential public health risk. One Health Outlook, 5: 2. https://doi.org/10.1186/s42522-023-00079-0
Reference
(Sarker et al., 2018)
Sarker MAR, Haque MM, Rifa RA, Ema FA, Islam MA and Khatun MM, 2018. Isolation and identification of bacteria from fresh guava (Psidium guajava) sold at local markets in Mymensingh and their antibiogram profile. Veterinary World, 11(8): 1145. https://doi.org/10.14202/vetworld.2018.1145-1149
Reference
(Hossain et al., 2014)
Hossain MT, Kim YR and Kong IS, 2014. PCR–restriction fragment length polymorphism analysis using groEL gene to differentiate pathogenic Vibrio species. Diagnostic Microbiology and Infectious Disease, 78: 9-11. https://doi.org/10.1016/j.diagmicrobio.2013.10.005
Reference
Bauer et al. (1966)
Bauer AW, Kirby WMM, Shrris JC and Truck M, 1966. Antibiotic susceptibility testing by a standardized single disk method. American journal of clinical pathology, 45(4): 493–496. https://cir.nii.ac.jp/crid/1571417124987473664
Reference
(CLSI, 2018)
CLSI, 2018. Performance standards for antimicrobial susceptibility testing. Wayne State University Press, Detroit, MI, USA. pp. 1–26.
Reference
(Mostafidi et al., 2020)
Mostafidi M, Sanjab MR, Shirkhan F and Zahedi MT, 2020. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends in Food Science and Technology, 103: 321-332. https://doi.org/10.1016/j.tifs.2020.07.009
Reference
(Nagyová et al., 2019)
Nagyová Ľ, Andocsová A, Géci A, Zajác P, Palkovič J, Košičiarová I and Golian J, 2019. Consumers´ awareness of food safety. Potravinarstvo Slovak Journal of Food Sciences, 13: 8–17. https://doi.org/10.5219/1003
Reference
(Sultana et al., 2021)
Sultana M, Limu S, Hosin KMK, Siddiqua A and Islam A, 2021. Isolation and identification of E Coli and V Cholerae from street fruits and juices from different areas in Dhaka city, Bangladesh. International Journal of Trend in Scientific Research and Development, 5(2): 789-793.
Reference
(Iwu and Okoh, 2019)
Iwu CD and Okoh AI, 2019. Preharvest transmission routes of fresh produce associated bacterial pathogens with outbreak potentials: A review. International Journal of Environmental Research and Public Health, 16(22): 4407. https://doi.org/10.3390/ijerph16224407
Reference
(Bilal et al., 2023)
Bilal H, Li X, Iqbal MS, Mu Y, Tulcan RXS and Ghufran MA, 2023. Surface water quality, public health, and ecological risks in Bangladesh—A systematic review and meta-analysis over the last two decades. Environmental Science and Pollution Research, 30(40): 91710-91728. https://doi.org/10.1007/s11356-023-28879-x
Reference
(Jeong et al., 2016)
Jeong H, Kim H and Jang T, 2016. Irrigation water quality standards for indirect wastewater reuse in agriculture: A contribution toward sustainable waste water reuse in South Korea. Water, 8(4): 169. https://doi.org/10.3390/w8040169
Reference
(Uyttendaele et al., 2015)
Uyttendaele M, Jaykus LA, Amoah P, Chiodini A, Cunliffe D, Jacxsens L and Rao Jasti P, 2015. Microbial hazards in irrigation water: standards, norms, and testing to manage use of water in fresh produce primary production. Comprehensive Reviews in Food Science and Food Safety, 14(4): 336-356. https://doi.org/10.1111/1541-4337.12133
Reference
(Yin et al., 2022)
Yin JH, Kelly PJ and Wang C, 2022. Flies as vectors and potential sentinels for bacterial pathogens and antimicrobial resistance: a review. Veterinary Sciences, 9(6): 300. https://doi.org/10.3390/vetsci9060300
Reference
(Wasala et al., 2013)
Wasala L, Talley JL, DeSilva U, Fletcher J and Wayadande A, 2013. Transfer of Escherichia coli O157: H7 to spinach by house flies, Musca domestica (Diptera: Muscidae). Phytopathology, 103(4): 373-380. https://doi.org/10.1094/PHYTO-09-12-0217-FI
Reference
Mahfuza et al. (2016)
Mahfuza I, Arzina H, Kamruzzaman MM, Afifa K, Afzal HM, Rashed N and Roksana H, 2016. Microbial status of street vended fresh-cut fruits, salad vegetables and juices in Dhaka city of Bangladesh. International Food Research Journal, 23(5): 2258.
Reference
Mrityunjoy et al. (2013 )
Mrityunjoy A, Kaniz F, Fahmida J, Shanzida JS, Aftab UM and Rashed N, 2013. Prevalence of V. cholerae in different food samples in the city of Dhaka, Bangladesh. International Food Research Journal, 20(2): 1017-1022.
Reference
Ratshilingano et al. (2022)
Ratshilingano MT, du Plessis EM, Duvenage S and Korsten L, 2022. Characterization of multidrug-resistant Escherichia coli isolated from two commercial lettuce and spinach supply chains. Journal of Food Protection, 85: 122–132. https://doi.org/10.4315/jfp-21-125
Reference
Datta et al. (2024)
Datta S, Ishikawa M, Chudhakorn S and Charaslertrangsi T, 2024. Prevalence and antimicrobial characteristics of Escherichia coli in selected vegetables and herbs in Bangkok, Thailand. Journal of Food Protection, 87(3): 100229. https://doi.org/10.1016/j.jfp.2024.100229
Reference
Rivera et al. (2001)
Rivera IN, Chun J, Huq A, Sack RB and Colwell RR, 2001. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Applied and Environmental Microbiology, 67(6): 2421-2429. https://doi.org/10.1128/AEM.67.6.2421-2429.2001
Reference
Singh et al. (2002)
Singh DV, Isac SR and Colwell RR, 2002. Development of a hexaplex PCR assay for rapid detection of virulence and regulatory genes in Vibrio cholerae. Journal of Clinical Microbiology, 40(12): 4266-4271. https://doi.org/10.1128/jcm.40.11.4321-4324.2002
Reference
Alabi et al. (2022)
Alabi OH, Obiekezie SO and Ekeleme K, 2022. Antibiotic resistance profiling and molecular detection of Escherichia coli Pathotypes isolated from irrigated fresh vegetables. AROC in Pharmaceutical and Biotechnology, 2: 18-26. https://doi.org/10.53858/arocpb02012735
Reference
Habib et al. (2023)
Habib I, Al-Rifai RH, Mohamed MYI, Ghazawi A, Abdalla A, Lakshmi G and Khan M, 2023. Contamination levels and phenotypic and genomic characterization of antimicrobial resistance in Escherichia coli isolated from fresh salad vegetables in the United Arab Emirates. Tropical Medicine and Infectious Disease, 8(6): 294. https://doi.org/10.3390/tropicalmed8060294
Reference
Dalsgaard et al. (2000)
Dalsgaard A, Forslund A, Serichantalergs O and Sandvang D, 2000. Distribution and content of class 1 integrons in different Vibrio cholerae O serogroups isolated in Thailand. Antimicrobial Agents and Chemotherapy, 44(5): 1315-1321. https://doi.org/10.1128/aac.44.5.1315-1321.20
Reference
Budiman et al. (2022)
Budiman A, Kurnia K and Waturangi DE, 2022. Prevalence and molecular characterization of Vibrio cholerae from fruits and salad vegetables sold in Jakarta, Indonesia, using most probable number and PCR. BMC Research Notes, 15: 63. https://doi.org/10.1186/s13104-022-05955-y
Reference
(Yu et al., 2019)
Yu P, Yu S, Wang J, Guo H, Zhang Y, Liao X and Ding Y, 2019. Bacillus cereus isolated from vegetables in China: incidence, genetic diversity, virulence genes, and antimicrobial resistance. Frontiers in microbiology, 10: 948. https://doi.org/10.3389/fmicb.2019.00948
References
Abioye OE, Osunla AC and Okoh AI, 2021. Molecular detection and distribution of six medically important Vibrio spp. in selected freshwater and brackish water resources in Eastern Cape Province, South Africa. Frontiers in Microbiology, 12: 617703. https://doi.org/10.3389/fmicb.2021.617703
Alabi OH, Obiekezie SO and Ekeleme K, 2022. Antibiotic resistance profiling and molecular detection of Escherichia coli Pathotypes isolated from irrigated fresh vegetables. AROC in Pharmaceutical and Biotechnology, 2: 18-26. https://doi.org/10.53858/arocpb02012735
Alam SN and Naser MN, 2020. Nutritional and health issues in Bangladesh and solutions through traditional foods. In: Nutritional and Health Aspects of Food in South Asian Countries (pp. 237-254). Academic Press. https://doi.org/10.1016/B978-0-12-820011-7.00026-5
Alegbeleye O and Sant’Ana AS, 2023. Microbiological quality of irrigation water for cultivation of fruits and vegetables: An overview of available guidelines, water testing strategies and some factors that influence compliance. Environmental Research, 220: 114771. https://doi.org/10.1016/j.envres.2022.114771
Alegbeleye O, Odeyemi OA, Strateva M and Stratev D, 2022. Microbial spoilage of vegetables, fruits and cereals. Applied Food Research, 2: 100122. https://doi.org/10.1016/j.afres.2022.100122
Aurin SA, Chowdhury SP, Abony M, Rifa J, Banik A, Fatema AN and Ahmed Z, 2020. Characterization of multi-drug resistant gram-negative bacteria present in fresh leafy and salad vegetables in Dhaka, Bangladesh. European Journal of Engineering and Technology Research, 5(11): 1322-1327. https://doi.org/10.24018/ejers.2020.5.11.2212
Aworh MK, Kwaga JK, Hendriksen RS, Okolocha EC, Harrell E and Thakur S, 2023. Quinolone-resistant Escherichia coli at the interface between humans, poultry and their shared environment-a potential public health risk. One Health Outlook, 5: 2. https://doi.org/10.1186/s42522-023-00079-0
Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J, 2018. Vibrio spp. infections. Nature Reviews Disease Primers, 4: 1-19. https://doi.org/10.1038/s41572-018-0005-8
Bauer AW, Kirby WMM, Shrris JC and Truck M, 1966. Antibiotic susceptibility testing by a standardized single disk method. American journal of clinical pathology, 45(4): 493–496. https://cir.nii.ac.jp/crid/1571417124987473664
Bekele F, Tefera T, Biresaw G and Yohannes T, 2017. Parasitic contamination of raw vegetables and fruits collected from selected local markets in Arba Minch town, Southern Ethiopia. Infectious Diseases of Poverty, 6: 1-7. https://doi.org/10.1186/s40249-016-0226-6
Bilal H, Li X, Iqbal MS, Mu Y, Tulcan RXS and Ghufran MA, 2023. Surface water quality, public health, and ecological risks in Bangladesh—A systematic review and meta-analysis over the last two decades. Environmental Science and Pollution Research, 30(40): 91710-91728. https://doi.org/10.1007/s11356-023-28879-x
Bolinches J, Romalde JL and Toranzo AE, 1988. Evaluation of selective media for isolation and enumeration of vibrios from estuarine waters. Journal of microbiological methods, 8(3): 151-160. https://doi.org/10.1016/0167-7012(88)90016-4
Budiman A, Kurnia K and Waturangi DE, 2022. Prevalence and molecular characterization of Vibrio cholerae from fruits and salad vegetables sold in Jakarta, Indonesia, using most probable number and PCR. BMC Research Notes, 15: 63. https://doi.org/10.1186/s13104-022-05955-y
Cheesbrough M, 1985. Medical laboratory manual for tropical countries. English Language Book Society, London. pp. 479.
Cheesbrough M, 2006. District laboratory practice in tropical countries. Cambridge University Press, Cambridge, UK. pp. 434.
Chowdhury F, Ross AG, Islam MT, McMillan NA and Qadri F, 2022. Diagnosis, management, and future control of cholera. Clinical Microbiology Reviews, 35(3): e00211-21. https://doi.org/10.1128/cmr.00211-21
CLSI, 2018. Performance standards for antimicrobial susceptibility testing. Wayne State University Press, Detroit, MI, USA. pp. 1–26.
Dalsgaard A, Forslund A, Serichantalergs O and Sandvang D, 2000. Distribution and content of class 1 integrons in different Vibrio cholerae O serogroups isolated in Thailand. Antimicrobial Agents and Chemotherapy, 44(5): 1315-1321. https://doi.org/10.1128/aac.44.5.1315-1321.20
Datta S, Ishikawa M, Chudhakorn S and Charaslertrangsi T, 2024. Prevalence and antimicrobial characteristics of Escherichia coli in selected vegetables and herbs in Bangkok, Thailand. Journal of Food Protection, 87(3): 100229. https://doi.org/10.1016/j.jfp.2024.100229
Desiree K, Schwan CL, Ly V, Hok L, Bello NM, Nwadike L and Vipham JL, 2021. Investigating Salmonella enterica, Escherichia coli, and coliforms on fresh vegetables sold in informal markets in Cambodia. Journal of Food Protection, 84(5): 843-849. https://doi.org/10.4315/JFP-20-219
Dias, 2012. Nutritional quality and health benefits of vegetables: A review. Food and Nutrition Sciences, 3(10): 1354-1374. https://doi.org/10.4236/fns.2012.310179
Ema FA, Shanta RN, Rahman MZ, Islam MA and Khatun MM, 2022. Isolation, identification, and antibiogram studies of Escherichia coli from ready-to-eat foods in Mymensingh, Bangladesh. Veterinary World, 15(6): 1497. http://doi.org/10.14202/vetworld.2022.1497-1505
Fleckenstein JM and Kuhlmann FM, 2019. Enterotoxigenic Escherichia coli infections. Current infectious disease reports, 21: 1-9. https://doi.org/10.1007/s11908-019-0665-x
Habib I, Al-Rifai RH, Mohamed MYI, Ghazawi A, Abdalla A, Lakshmi G and Khan M, 2023. Contamination levels and phenotypic and genomic characterization of antimicrobial resistance in Escherichia coli isolated from fresh salad vegetables in the United Arab Emirates. Tropical Medicine and Infectious Disease, 8(6): 294. https://doi.org/10.3390/tropicalmed8060294
Haque MM and Hoque MZ, 2021. Vegetable production and marketing channels in Bangladesh: Present scenario, problems, and prospects. In Seminar Paper.
Hossain MS, Jony MAH, Saha N, Islam B, Sobur KA, Mowdood S and Hossain KM, 2024. Antibiotic Resistance Genes Detection in Escherichia coli Isolated from Raw Meat in Rajshahi Division of Bangladesh. American Journal of Microbiological Research, 12(4): 79-84. https://doi.org/10.12691/ajmr-12-4-1
Hossain MT, Kim YR and Kong IS, 2014. PCR–restriction fragment length polymorphism analysis using groEL gene to differentiate pathogenic Vibrio species. Diagnostic Microbiology and Infectious Disease, 78: 9-11. https://doi.org/10.1016/j.diagmicrobio.2013.10.005
Islam S, Thangadurai D, Sangeetha J and Cruz-Martins N, 2023. Global food safety: Microbial interventions and molecular advancements. CRC Press. pp. 366.
ISO, 1995. Recommendation of the meeting of the subcommittee, international organization for standardization on meat and meat products. ISO/TC-36/ SC 6.10-18. ISO, Geneva, Switzerland.
Iwu CD and Okoh AI, 2019. Preharvest transmission routes of fresh produce associated bacterial pathogens with outbreak potentials: A review. International Journal of Environmental Research and Public Health, 16(22): 4407. https://doi.org/10.3390/ijerph16224407
Jeong H, Kim H and Jang T, 2016. Irrigation water quality standards for indirect wastewater reuse in agriculture: A contribution toward sustainable waste water reuse in South Korea. Water, 8(4): 169. https://doi.org/10.3390/w8040169
Julqarnain SM, Bose P, Rahman MZ, Khatun MM and Islam MA, 2022. Bacteriological quality and prevalence of foodborne bacteria in broiler meat sold at live bird markets at Mymensingh City in Bangladesh. Journal of Advanced Veterinary and Animal Research, 9(3): 405. http://doi.org/10.5455/javar.2022.i608
Łepecka A, Zielińska DS, zymański P, Buras I and Kołożyn-Krajewska D, 2022. Assessment of the microbiological quality of ready-to-eat salads—are there any reasons for concern about public health? International Journal of Environmental Research and Public Health, 19(3): 1582.https://doi.org/10.3390/ijerph19031582
Machado‐Moreira B, Richards K, Brennan F, Abram F and Burgess CM, 2019. Microbial contamination of fresh produce: what, where, and how?Comprehensive reviews in food science and food safety, 18(6): 1727-1750. https://doi.org/10.1111/1541-4337.12487
Mahfuza I, Arzina H, Kamruzzaman MM, Afifa K, Afzal HM, Rashed N and Roksana H, 2016. Microbial status of street vended fresh-cut fruits, salad vegetables and juices in Dhaka city of Bangladesh. International Food Research Journal, 23(5): 2258.
Mostafidi M, Sanjab MR, Shirkhan F and Zahedi MT, 2020. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends in Food Science and Technology, 103: 321-332. https://doi.org/10.1016/j.tifs.2020.07.009
Mrityunjoy A, Kaniz F, Fahmida J, Shanzida JS, Aftab UM and Rashed N, 2013. Prevalence of V. cholerae in different food samples in the city of Dhaka, Bangladesh. International Food Research Journal, 20(2): 1017-1022.
Nagyová Ľ, Andocsová A, Géci A, Zajác P, Palkovič J, Košičiarová I and Golian J, 2019. Consumers´ awareness of food safety. Potravinarstvo Slovak Journal of Food Sciences, 13: 8–17. https://doi.org/10.5219/1003
Ongeng D, Geeraerd AH, Springael D, Ryckeboer J, Muyanja C and Mauriello G, 2015. Fate of Escherichia coli O157: H7 and Salmonella enterica in the manure-amended soil-plant ecosystem of fresh vegetable crops: A review. Critical reviews in microbiology, 41(3): 273-294. https://doi.org/10.3109/1040841X.2013.829415
Osafo R, Balali GI, Amissah-Reynolds PK, Gyapong F, Addy R, Nyarko AA and Wiafe P, 2022. Microbial and parasitic contamination of vegetables in developing countries and their food safety guidelines. Journal of Food Quality, 2022: 4141914. https://doi.org/10.1155/2022/4141914
Pinto T, Aires A, Cosme F, Bacelar E, Morais MC, Oliveira I, Gonçalves B, 2021. Bioactive (poly) phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods, 10: 106. https://doi.org/10.3390/foods10010106
Qamar MU, Rizwan M, Uppal R, Khan AA, Saeed U, Ahmad K and Suleman M, 2023. Antimicrobial susceptibility and clinical characteristics of multidrug-resistant polymicrobial infections in Pakistan, a retrospective study 2019–2021. Future Microbiology, 18(17): 1265-1277. https://doi.org/10.2217/fmb-2023-0110
Ratshilingano MT, du Plessis EM, Duvenage S and Korsten L, 2022. Characterization of multidrug-resistant Escherichia coli isolated from two commercial lettuce and spinach supply chains. Journal of Food Protection, 85: 122–132. https://doi.org/10.4315/jfp-21-125
Rebaudet S, Sudre B, Faucher B and Piarroux R, 2013. Environmental determinants of cholera outbreaks in inland Africa: a systematic review of main transmission foci and propagation routes. The Journal of Infectious Diseases, 208: S46-S54. https://doi.org/10.1093/infdis/jit195
Rivera IN, Chun J, Huq A, Sack RB and Colwell RR, 2001. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Applied and Environmental Microbiology, 67(6): 2421-2429. https://doi.org/10.1128/AEM.67.6.2421-2429.2001
Rudzińska A, Juchaniuk P, Oberda J, Wiśniewska J, Wojdan W, Szklener K, Mańdziuk S, 2023. Phytochemicals in cancer treatment and cancer prevention—review on epidemiological data and clinical trials. Nutrients, 15(8): 1896. https://doi.org/10.3390/nu15081896
Said DES, 2012. Detection of parasites in commonly consumed raw vegetables. Alexandria Journal of Medicine, 48(4): 345-352. https://doi.org/10.1016/j.ajme.2012.05.005
Sarker MAR, Haque MM, Rifa RA, Ema FA, Islam MA and Khatun MM, 2018. Isolation and identification of bacteria from fresh guava (Psidium guajava) sold at local markets in Mymensingh and their antibiogram profile. Veterinary World, 11(8): 1145. https://doi.org/10.14202/vetworld.2018.1145-1149
Silva Dias JC, 2022. Nutritional quality and health benefits of vegetables. Emerging Trends in Disease and Health Research, 4: 7–35. https://doi.org/10.9734/bpi/etdhr/v4/15660D
Singh DV, Isac SR and Colwell RR, 2002. Development of a hexaplex PCR assay for rapid detection of virulence and regulatory genes in Vibrio cholerae. Journal of Clinical Microbiology, 40(12): 4266-4271. https://doi.org/10.1128/jcm.40.11.4321-4324.2002
Sotler R, Poljšak B, Dahmane R, Jukić T, Pavan Jukić D, Rotim C and Starc A 2019. Prooxidant activities of antioxidants and their impact on health. Acta Clinica Croatica, 58(4): 726-736. https://doi.org/10.20471/acc.2019.58.04.20
Sultana M, Limu S, Hosin KMK, Siddiqua A and Islam A, 2021. Isolation and identification of E Coli and V Cholerae from street fruits and juices from different areas in Dhaka city, Bangladesh. International Journal of Trend in Scientific Research and Development, 5(2): 789-793.
Uyttendaele M, Jaykus LA, Amoah P, Chiodini A, Cunliffe D, Jacxsens L and Rao Jasti P, 2015. Microbial hazards in irrigation water: standards, norms, and testing to manage use of water in fresh produce primary production. Comprehensive Reviews in Food Science and Food Safety, 14(4): 336-356. https://doi.org/10.1111/1541-4337.12133
Wasala L, Talley JL, DeSilva U, Fletcher J and Wayadande A, 2013. Transfer of Escherichia coli O157: H7 to spinach by house flies, Musca domestica (Diptera: Muscidae). Phytopathology, 103(4): 373-380. https://doi.org/10.1094/PHYTO-09-12-0217-FI
Weil AA and Harris JB, 2015. V. cholerae. In: Molecular medical microbiology (pp. 1079-1098). Academic Press. https://doi.org/10.1016/B978-0-12-397169-2.00060-3
Yin JH, Kelly PJ and Wang C, 2022. Flies as vectors and potential sentinels for bacterial pathogens and antimicrobial resistance: a review. Veterinary Sciences, 9(6): 300. https://doi.org/10.3390/vetsci9060300
Yu P, Yu S, Wang J, Guo H, Zhang Y, Liao X and Ding Y, 2019. Bacillus cereus isolated from vegetables in China: incidence, genetic diversity, virulence genes, and antimicrobial resistance. Frontiers in microbiology, 10: 948. https://doi.org/10.3389/fmicb.2019.00948
How to cite
Rana AH, Bose P, Sobur KA, Hossen MM, Mowdood S, Hossain MK, Afroz F, Rumi NA, Hasan M, Jahan N, Titas AR and Hosen MA 2024. Molecular detection and antibiogram profiles of Escherichia coli and Vibrio cholerae isolated from raw vegetables in the northern district of Bangladesh. Journal of Bioscience and Environment Research, 1(1): 26-34. https://doi.org/10.69517/jber.2024.01.01.0006



