The proliferation of Salmonella sp. in river water and soil exemplifies the contamination of aquatic environments. Indubitably, contaminated water bodies are major sources of infectious diseases, causing suffering among residents inhabiting riverside areas. The prevalent reservoir of Salmonella is the gastrointestinal tract of many animals, including birds, reptiles, livestock and humans. Once released from the gastrointestinal tract through feces or exudates, Salmonella can be conveyed to water bodies through rainfall and surface runoff, highlighting its persistence in the environment. As a significant human pathogen, Salmonella is responsible for salmonellosis, which encompasses acute diarrheal disease, enteric fever, gastroenteritis, abdominal pain and vomiting. The prevalence of Salmonella sp. is a major global public health concern, with an estimated 93.8 million cases of illness and 155,000 deaths annually due to salmonellosis (Liu et al., 2018). Despite this alarming prevalence, the specific pathways through which Salmonella contaminates local water sources remain poorly understood, underscoring the urgent need for further investigation.
Salmonella is a rod-shaped, flagellated, gram-negative facultative anaerobe that belongs to the family Enterobacteriaceae. According to the Centers for Disease Control and Prevention (CDC) nomenclature system, the genus Salmonella contains only two species—S. enterica and S. bongori—based on differences in their 16S rRNA sequence analysis. Notably, Salmonella enterica subsp. enterica accounts for approximately 99% of Salmonella infections in humans and warm-blooded animals, with over 1,400 serotypes identified. Among these, S. enterica subsp. enterica serotypes typhi and paratyphi are predominantly transmitted through water, causing enteric fever, while S. enterica subsp. enterica serotype Typhimurium has a broad host range and primarily causes gastroenteritis. This longstanding disease, resulting from S. typhi and Salmonella enterica serovar paratyphi (S. paratyphi), was among the deadliest during the time before antibiotics were available (Saha et al., 2018). Studies indicate that enteric fever results in 2.9 million cases and 95,000 deaths globally, with about 200 cases per 100,000 person-years during the period of 2003 to 2004 (Naheed et al., 2010).
The lifeline and major watercourse of the Chattogram region, the largest port city on the southeastern coast of Bangladesh, the Karnaphuli River plays a vital role in agribusiness, industries, and the necessities of local communities and wildlife. Approximately 300 industries, including 100 ship-breaking operations, 19 tanneries and 26 textile factories, discharge waste near the Karnaphuli River (Uddin and Jeong, 2021; Hossain, 2006). The Department of Environment (DoE) has reported that more than 350 metric tons of toxic substances are being released into the Karnaphuli River daily (Hossain, 2006). Additionally, 5,000 tons of household wastes are discharged into the river every day through 36 canals of Chattogram city, containing pathogenic microorganisms, including Salmonella sp., thereby polluting surface water and sediment. This rampant contamination raises critical concerns regarding the persistence of Salmonella sp. in these waters, posing significant health risks to the local population dependent on this water source.
Moreover, industrial waste contains high concentrations of heavy metals, leading to stress factors that promote the formation of biofilms by Salmonella (Sallami et al., 2022). Such biofilms, complex surface-associated communities, are implicated in approximately 80% of human bacterial infections (Borges et al., 2018). Pollution from industrial effluents and household waste destabilizes physiochemical parameters, with the DoE reporting that many characteristics of the Karnaphuli River fall below recommended standards, affecting the presence and persistence of Salmonella sp. (Uddin and Jeong, 2021). The use of contaminated water for drinking, household or recreational purposes, as well as the use of riverbank sediment for agriculture, poses severe health risks. The World Health Organization and the Environment Quality Standard for Bangladesh stipulate that the standard value for Salmonella sp. in drinking water should be nil/ml, yet the Karnaphuli River contains ~18,000 coliforms/100 ml near sewage disposal areas, indicating significant contamination (Dey et al., 2017).
The levels of Salmonella sp. in the water and sediment of the Karnaphuli River are expected to be significantly higher in areas with greater pollution loads, which may correlate with increased incidences of waterborne diseases among the local population. While existing research has addressed the contamination of the Karnaphuli River, comprehensive studies investigating the persistence and ecological dynamics of Salmonella sp. in both water and sediment are still lacking, particularly concerning industrial pollution and its impact on public health. This study aims to evaluate the persistence of Salmonella sp. in the water and sediment of the Karnaphuli River, providing critical insights into its environmental dynamics. The findings will enhance the understanding of Salmonella's role in waterborne disease transmission and may inform local health policies and pollution management strategies, ultimately promoting public health and environmental sustainability in the Chattogram region.
2. Materials and Methods
2.1 Ethical approval
No ethical approval is required for this study.
2.2 Sampling area
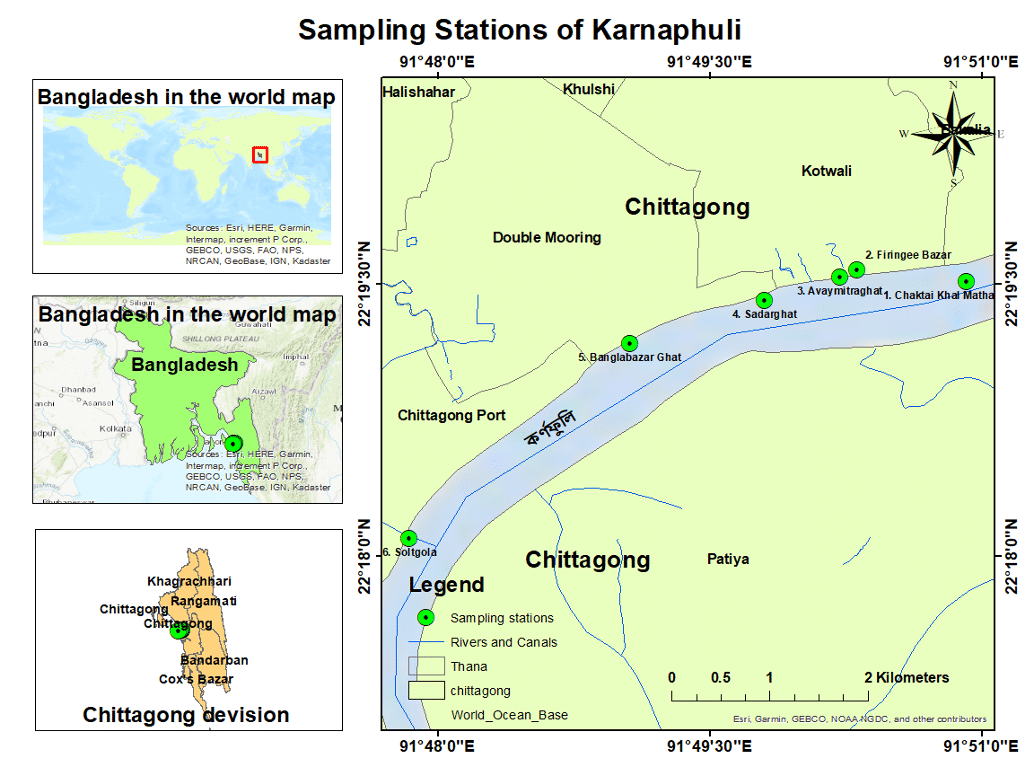
The study was conducted in Barishal Sadar Upazila, Barishal district, Bangladesh from November 2021, to January 2022 (Figure 1). The study population consisted of goats, and data were collected from the Upazila livestock office and veterinary hospital, involving 216 goats in total.
Table 1. Description and GPS coordinates of the sampling points.
The six selected sampling stations are known as Chaktai Khal Matha, Firingee Bazar Ghat, Avaymitra Ghat (which is close to the most polluted water channel in Chittagong city known as Rajakhal), Sadarghat, Bangla Bazar ghat and Saltgola respectively showed in Figure 1.
There are about 300 factories and industrial establishments along the 100-kilometer area of the Karnaphuli extending from Chandraghona to the mouth of the river at Patenga, including cement factories, fertilizer manufacturers, paper mills, rayon mills, tanneries, oil refineries, power plants, dyeing and washing plants (Bhuyan and Islam, 2017). Among 36 canals that are connected to the Karnaphuli river, some significant canals known as Mohesh Khal, Chaktai Khal, Murari Khal, Khondokia Khal, Boalkhali canal, BoRubi Gate Khal, Maizpara Khal, and Rajakhali Khal, dump raw and partially decomposed sewages, solid wastes, domestic organic wastes and untreated industrial discharge into the Karnaphuli river (Hossain et al., 2006).
Chaktai Khal, also known as the grief of Chittagong City, plays the most dominant role in the environment of the Karnaphuly River by dumping a large amount of solid and liquid wastes from the industrial, commercial, and residential areas adjacent to it. Again, the Saltgola canal contains a high amount of chemical waste from CEPZ and port-based industries which contain many heavy mineral particles. Fish wastage, human wastage, oil spills of fishing vessels, market waste, etc. also caused pollution at Firingee Bazar Ghat and Sadarghat area of Karnaphuli River. Besides, there are 400 slaughterhouses in the Firingee Bazar and Dewanhat areas alone. The blood from the slaughtered animals directly finds its way into the river. Moreover, about 50,000 sanitary and 24,000 unhygienic traditional latrines of the port city are directly linked to the study area of this river (Islam et al., 2016).
2.3 Sample collection and analysis with preservation
The present work was carried out in September 2021 just after heavy rain. Water and soil samples were collected from six different stations of the Karnaphuli River estuary after extreme high tide when low tide started during this study period. In situ measurement water temperature was measured by a centigrade thermometer where water pH and salinity were measured by a pen pH meter and refractometer respectively. Dissolve Oxygen (DO) concentration was measured in the laboratory by the standard method (APHA, 2017). Water samples from three different stations were collected from 500 ml. bottles from the Karnaphuli River estuary with screw-capped and sealed with aluminum foil paper to control the bacterial growth before proceeding in an ice box at about 4 ºC temperature until brought to the laboratory of the Department of Oceanography, CU. Soil samples were also collected from six same stations with grab samplers and stored in 500 g jars. During the sampling, at first, the grab samplers were throughout to the bottom which carries a lot of soil filled with jars and then capped under an icebox and brought to the specified laboratory.
2.4 Bacterial isolation and enumeration
The diversity of pathogenic bacteria (Salmonella sp.) in water and soil was identified by standard plate count (SPC) techniques (Some et al., 2021). The above methods for enumeration of pathogenic bacteria were because each of them gives characteristics type of colony confirmed by further gram straining, biochemical, and fermentation tests for accuracy.
As we are searching for Salmonella sp., we used specific agar media Bismuth sulfite agar (BSA) particularly useful for the isolation of lactose-fermenting Salmonellae. S. typhi, S. enteritidis, and S. typhimurium typically grow as black colonies with a surrounding metallic sheen resulting from hydrogen sulfide production and reduction of sulfite to black ferric sulfide.
For isolation from the water sample, aseptically 1 ml suspension from diluted suspensions of 10-1 and 10-2 was poured into BSA agar plates for isolation of Salmonella sp. and gently rotated for spreading the suspension. Agar plates were incubated for 24 hours at 37 °C. In the case of sediment, 1 g sediment from each sample was suspended into 9 ml sterile distilled water and serially diluted to suspensions of 10-2, and 1 ml suspension from 10-1 and 10-2 dilution was poured into BSA agar plates for isolation and incubated for 48 hours in 37 °C. After 24 hours, all the black colonies with a surrounding metallic sheen are counted. The number of colonies is multiplied by the dilution factor and the mean result from dilution 10-1 and 10-2 plates is recorded. The cultures were identified according to the bacteriological analytical manual (BAM) of the USFDA. 2 colonies from Petri dishes are further tested (gram staining, Indole test, methyl red test, urease test Voges Proskauer test, starch hydrolysis test, catalase activity test, and fermentation test) for confirmation of Salmonella sp.
2.5 Data analysis
The relation between the physical parameters and Salmonella sp. from water and sediment are statistically analyzed with R programming and visualized with the correlogram showing similarity and dissimilarity among the parameters along with Salmonella sp. The result and the spatial distribution of Salmonella sp. are visualized with ArcGIS (10.8).
3. Results and Discussion
Assembled samples were collected from the surface water and sediment at the interval time prevailed ending of high tide and the beginning of low tide (Table 1).
Table 2. Different types of parameters, HT-LT= high tide to low tide.
In a detailed comparative assessment of water and sediment samples across six stations (St-1 to St-6), a consistent tidal situation of HT-LT (after extreme high tide when low tide started) was noted for most samples, with stations St-5 and St-6 presenting data near LT. Surface water samples predominantly exhibited alarming signals of environmental distress: the odor ranged from pungent to extremely pungent; visual inspection revealed colors from muddy to oily black; and all stations, except St-5, presented extremely to highly polluted conditions. Temperature values hovered around 30 °C, with salinity fluctuating between 0.9 to 5.8 ppt and DO levels varying from 3.3 to 5.06 mg/L. Notably, St-6 had a remarkably higher salinity of 5.8 ppt. The pH levels remained consistent, averaging around 7.4, and the Ec, intriguingly, soared to 8 mS/m at St-6. In the sedimentary analysis, all stations demonstrated profound pollution levels, with colors ranging from blackish to brownish. The depth of these sediment samples varied from 1.82 to 4.57 m, and all exhibited a lack of salinity, DO, and an average pH value lower than that of water samples, suggesting distinct yet alarming ecological disparities between water and sediment profiles (Bhuyan and Islam, 2017).
In an evaluation of water quality parameters across six distinct stations, temperature remained relatively consistent, ranging from 28.9 °C to 30.6 °C, suggesting minimal thermal variation across the sites. Salinity displayed a more diverse profile, with values ranging from 0.9 ppt at Firingee Bazar to 5.8 ppt at Soltgola. Dissolved oxygen levels fluctuated between 3.3 mg/L and 5.06 mg/L, but no discernible correlation with Salmonella sp. concentrations was evident. Notably, pH measurements were predominantly neutral, barring an outlier at Soltgola with an elevated pH of 8. Electrical conductivity was largely consistent, yet an anomalous value of 8 mS/m at Soltgola warrants further investigation. Preliminary observations do not indicate a direct correlation between the assessed physicochemical parameters and Salmonella sp. Counts (Counts are given in supplementary file as table), necessitating a more expansive dataset for robust statistical analysis (Hossain et al., 2006).
In analyzing the interdependencies among environmental parameters positive correlations are depicted in shades of blue, while negative correlations are represented by shades of red (Figure 2).
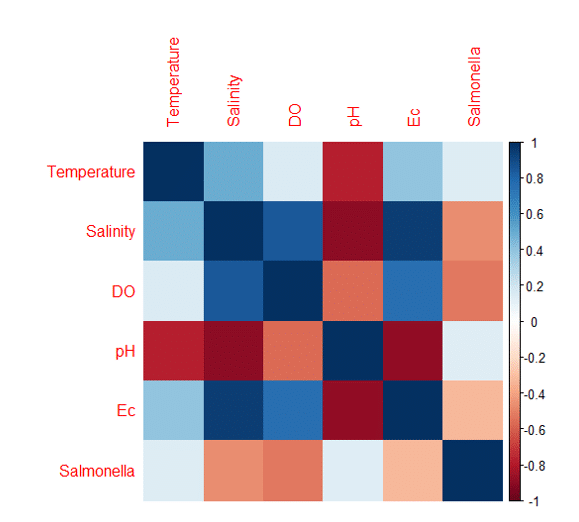
We observe the temperature exhibits a positive moderate correlation with salinity (r=0.497) suggesting that rising temperatures might be concomitant with increasing salinity levels. Additionally, there's a subtle positive association with both DO (r=0.158) and Ec (r=0.399), whereas a notably strong negative correlation is discerned with pH (r=-0.772). The relationship between temperature and Salmonella prevalence is weak yet positive (r=0.147). Salinity shows a particularly strong positive linkage with DO (r=0.848) and Ec (r=0.948), while manifesting a pronounced negative correlation with pH (r=-0.895) and a moderate one with Salmonella (r=-0.467). DO levels inversely correlate with pH (r=-0.567) and directly with Ec (r=0.752), and a decrease in Salmonella prevalence appears concomitant with increasing DO levels (r=-0.527). Furthermore, pH values and Ec illustrate a strong inverse relationship (r=-0.881) and only a marginal positive association between pH and Salmonella is observed (r=0.133). Lastly, an increase in Ec seems to be mildly associated with a decrease in Salmonella presence (r=-0.327). As expected, all parameters correlate perfectly with themselves (r=1.0). This comprehensive analysis provides critical insights into the intricate relationships among the evaluated environmental parameters (Some et al., 2021).
The status of Salmonella sp. in sediment and water sample is shown in Figures 3 and 4. Salmonella sp. is found more in water than in sediment. In the case of water, the 1st and 2nd stations Chaktai Khal matha and Firingee Bazar are mostly threatened by the colossal concentration of Salmonella sp. due to their locations and hazardous condition already described in sampling area. In between these two sites, the 2nd station, Firingee Bazar is the dominant reservoir of Salmonella sp. containing 1725 cells/ml. Among the six stations, the 5th and 6th stations comprise the lowest concentration of Salmonella sp. containing 250 cells/ml, significantly higher than the recommended standard level.
In the case of sediment, 1st and 2nd stations have a high concentration of bacteria whereas 2nd station holds 365 cells/ml of Salmonella sp. which is the highest concentration among the six stations.

The morphological, biochemical, and fermentation studies conducted on Salmonella sp. from water and soil samples of the Karnaphuli River confirmed its presence in both environments. Morphological analysis using Bismuth Sulfite Agar showed characteristic black colonies, with Gram staining identifying the bacteria as short, rod-shaped, and arranged singly, indicative of Salmonella sp. (Table 2).
Biochemical tests revealed that the isolates were Gram-negative, methyl red-positive, and indole, Voges-Proskauer, urea, catalase, and starch hydrolysis-negative, consistent with the known traits of Salmonella (Table 3).

Table 3. Morphological tests and studies of Salmonella sp.
Table 4. Bio-chemical test results of bacteria colony.
Fermentation tests showed variable gas and acid production, with both samples fermenting glucose, maltose, and mannitol, while lactose was not fermented (Table 4). These findings confirm the pathogenic nature of Salmonella sp. and its potential threat to public health due to the environmental contamination of the river (Liu et al., 2018).
Table 5. Fermentation test result of bacteria colony.
4. Conclusions
The once-mighty river Karnaphuli, which shaped the lives of its bank dwellers, now harbors life-threatening pathogenic Salmonella sp. This study demonstrated that high concentrations of Salmonella sp. were found in both the water and sediment of the Karnaphuli River, indicating a severe public health threat associated with the consumption of water and soil from this river. The health implications for the riverside residents are alarming, as many suffer from various Salmonella infections, including acute diarrhea, enteric fever and abdominal pain. Throughout the study period, detected bacterial concentrations of Salmonella sp. in both water and sediment exceeded standard levels at all sampling stations. The growth of nutrient overflow and land runoff has contributed to a marked increase in Salmonella colony growth, reflecting the deteriorating status of the river. While several investigations have been conducted on various water bodies within the Karnaphuli system, further research is crucial to comprehensively assess the presence of diverse waterborne pathogens. If unaddressed, the situation in the Karnaphuli River area and broader Chattogram region will lead to substantial public health challenges due to salmonellosis, as the river water becomes increasingly unsuitable for consumption and other uses. Addressing these issues is essential for safeguarding the health of local communities and restoring the integrity of this vital waterway.
Acknowledgements
This research work was supported by the ‘Research and Publication Office’ financial grant from the University of Chittagong (CU), Bangladesh. The authors are also grateful for the technical and instrument support of the Department of Oceanography, CU to complete the work successfully. The authors also accord their obliged admiration to my research students for their supervision, helpful suggestions, and earnest cooperation in carrying out the study.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Informed consent statement
Not required.
Conflict of interest
The authors report there are no competing interests to declare.
Author contributions
Conceptualization: Md. Wahidul Alam and Md. Atiqul Islam Mondal; Data collection: All authors involved in data collection; Data analysis: Md. Jahin Khandakar; Figure preparation: Md. Jahin Khandakar and Fowzia Aktar; Manuscript writing and editing: Most. Israt Jahan, MD. Mosfiqur Rahman and Fowzia Aktar. All authors critically reviewed the manuscript and agreed to submit the final version of the manuscript.




