The propagation of finfishes through artificial reproduction has become an integral part of aquaculture research and commercial fisheries management (Islam et al., 2016; Rahman et al., 2016). As demand for fish protein increases globally, reliable and efficient breeding techniques are essential to support the sustainable cultivation of aquatic species. Induced breeding using synthetic hormones has become a widely accepted method to facilitate ovulation and spermiation in many freshwater and marine fish species (Ali et al., 2016a; Ali et al., 2016b; Zamri et al., 2022). Hormone preparations are now distributed commercially, enabling researchers and fish breeders to conduct experiments on various species, including those that have received limited scientific attention (Ljubobratović et al., 2021; Xu et al., 2023; Yeasmin et al., 2018). The advancement in synthetic hormone technology has led to the replacement of traditional agents like Carp Pituitary Extract (CPE) with more efficient and consistent alternatives, such as Human Chorionic Gonadotropin (HCG), Gonadotropin-Releasing Hormone Analogs (GnRH-a), Luteinizing Hormone-Releasing Hormone Analogs (LHRHA), and Domperidone (DOM) (Ashraf et al., 2024; Kucharczyk et al., 2020). The LHRHA is a synthetic peptide that mimics the native structure of naturally occurring LHRH. LHRHA acts as a potent stimulator of gonadotropin release, thereby promoting gamete maturation and inducing spawning in many teleost fish (Mohammadzadeh et al.,2020). It is often marketed as a versatile hormone for a wide variety of fish species, with recommended dosages typically ranging between 20–70 μg/kg body weight of broodstock (Khatun et al., 2024; Shabuj et al., 2016). However, its efficiency in certain species can be inconsistent, primarily when used without additional agents, such as dopamine antagonists, which help counteract the inhibitory effect of dopamine on the hypothalamic-pituitary-gonadal axis (Dairaghi et al., 2022). In contrast, Ovaprim, a commercially available hormone preparation, has emerged as a widely used alternative in induced breeding protocols. Ovaprim is a liquid formulation comprising Salmon Gonadotropin-Releasing Hormone Analog (sGnRH-a) at a concentration of 20 μg/mL and DOM at 10 mg/mL (Zadmajid et al., 2017). The inclusion of Domperidone, a dopamine antagonist, enhances the efficacy of Ovaprim by overcoming the inhibitory effects of dopamine, ensuring reliable induction of ovulation and spermiation (Al Adawiyah et al., 2019; Zadmajid, 2016). Ovaprim has been extensively tested across a variety of freshwater and marine fish species, proving effective and easy to administer due to its stable, pre-mixed liquid form (Chaube et al., 2014; DiMaggio et al., 2013). The Bornean Spotted Barb (Puntius sealei), a member of the Cyprinidae family, is a small to medium-sized freshwater fish endemic to the island of Borneo. It is recognized by its distinct appearance, characterized by four prominent black spots on its midsection and additional spots at the base of the dorsal and anal fins. Despite its incidental economic importance, P. sealei remains under-studied, particularly with regard to its reproductive biology and artificial propagation. The species is commonly harvested for food, where it is either consumed fresh or processed into “Kasam Ikan,” a local fermented delicacy. While P. sealei is listed as “Least Concern” under the IUCN Red List, increasing exploitation of wild populations for food and ornamental purposes underscores the importance of developing breeding protocols for conservation and sustainable utilization (Abit et al., 2021; Astuti et al., 2023). Previous studies on P. sealei have provided foundational information on its induced breeding and early embryonic development. Study also suggested that a successful induced spawning using Ovaprim at doses of 0.5 mL/kg for females and 0.25 mL/kg for males, reporting high fertilization and hatching rates under controlled conditions (Abit et al., 2021). However, no comparative studies have been conducted to evaluate the effectiveness of other synthetic hormones, such as LHRHA, on spawning induction in this species. Since LHRHA is a widely available and cost-effective hormone, understanding its potential utility in P. sealei breeding would provide valuable insights for hatchery operators and researchers. The aim of the present study was to compare the effectiveness of two synthetic hormones, LHRHA and Ovaprim, in inducing ovulation and spermiation in P. sealei. The study focused on determining the spawning success, fertilization rates, and early embryonic development of eggs produced using the two hormone treatments. By comparing the outcomes of LHRHA and Ovaprim, this study sought to identify the more effective hormone for P. sealei breeding, while also exploring potential factors that may influence the efficacy of each hormone. The findings of this research hold significance for the sustainable management and artificial propagation of P. sealei. Additionally, the study contributes to the broader understanding of synthetic hormone applications in fish reproduction, providing a basis for future studies on related species with similar biological and ecological characteristics.
2. Materials and Methods
2.1 Ethical approval
No ethical approval was required to conduct the study.
2.2 Experimental animals
A total number of 12 pairs (24 fish) of mature P. sealei were obtained from the Aquatics Wet Lab, Faculty of Resources Science and Technology UNIMAS Campus, Malaysia (Figure 1).
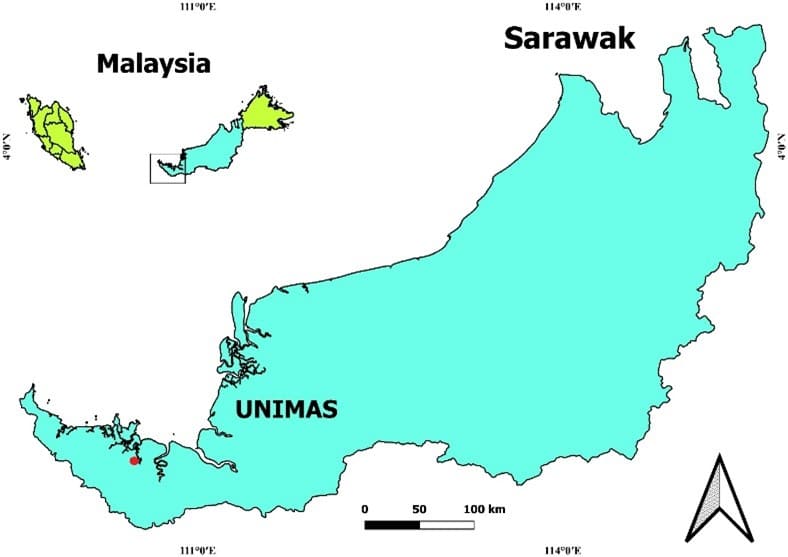 Figure 1. The study was conducted in the laboratory of UNIMAS, Malaysia.
Figure 1. The study was conducted in the laboratory of UNIMAS, Malaysia. 2.3 Broodstock selection
Only mature broodstock were selected for the breeding trial. The mature male and female broodstocks were selected from a wide pool of captive specimens available at the Aquatics Wet Lab, Faculty of Resources Science and Technology UNIMAS Campus. All fish selected were in healthy condition and of mature size (TL>8CM). The selected broodstock weighed between 85 to 170 grams in body weight. Prior to hormone treatment the fish were segregated by sex into two 1000 litre HDPE tanks equipped with aeration. Hormonal injections were administered after allowing the experimental animals to acclimatize for 24 hours to hatchery conditions.
2.4 Hormone selection
Two synthetic hormones (Ovaprim and LHRHA) (Syndel, Canada) were selected for comparison in this study. Hormonal stimulation was performed with two preparations Ovaprim (D-Arg6, Pro9-Net-sGnRH) and LHRA homogenized in a 0.9% NaCl solution. Fish were injected intramuscularly at the base of the dorsal fin at dosages presented in Table 1.
Table 1. Dosage of hormone preparation applied in the reproductive trial of Puntius sealei.
After 18 hours the tanks of each spawning pair was investigated for fertilized eggs. Any eggs produced were collected and examined under dissecting microscope to determine fertilization. Fertilized eggs were incubated in hatching trays. The influence of stimulants from each experimental treatment on the biological quality of gametes was determined by observing egg samples under microscope till hatching. Survival rate was determined by subtracting the volumetrically estimated total hatching larvae from total estimated egg count.
2.5 Embryonic and larval development observation
The developing embryonic stages of fertilized eggs were observed under magnification (compound microscope: LEICA zoom 2000) at 1 hour intervals until hatching.
2.6 Statistical analysis
All data collected, including spawning success, fertilization rate, and hatching rate, were analyzed using descriptive statistics (mean and standard deviation). Comparative analysis between the two hormone treatments (LHRHA and Ovaprim) was conducted to evaluate differences in spawning success and embryonic development. Spawning success was expressed as the percentage of female fish that ovulated successfully. Fertilization rate was calculated as the percentage of fertilized eggs out of the total number of eggs produced, while hatching rate was expressed as the proportion of eggs that successfully hatched into larvae. The spatial map of the study area was prepared using QGIS version 3.34.
3. Results and Discussion
Ovulation was confirmed in 83.3% of the female Puntius sealei (5 individuals) stimulated with Ovaprim, 75% of the spawned eggs were fertilized and underwent embryogenesis and embryos developed normally and hatched within 24 hours of fertilization (Figures 2 to 6). None of the paired fish in the group stimulated with LHRHA spawned. Ambient temperatures ranged from 27 to 29 °C. Ovaprim-treated females successfully spawned, with a substantial portion of the eggs being fertilized and progressing through normal embryogenesis. The absence of abnormal development during this process indicates that Ovaprim not only triggered ovulation but also supported viable reproductive outcomes (Nargesi et al., 2022, 2023). In contrast, LHRHA-treated pairs did not exhibit any spawning behavior, suggesting a limitation in its ability to stimulate reproductive processes in this species under the conditions of the current study. The success of Ovaprim can be attributed to its formulation, which combines a SGnRHA with a dopamine antagonist, domperidone. This synergistic combination is crucial for overcoming the inhibitory effects of dopamine, which otherwise suppress the natural release of gonadotropins in fish. By counteracting dopamine’s inhibitory role, Ovaprim effectively promotes the release of luteinizing hormone, leading to gamete maturation and ovulation. This mechanism has been widely documented across various teleost species and explains the consistent success of Ovaprim in artificial breeding trials (Acharjee et al., 2017; Zadmajid et al., 2017). In contrast, LHRHA, despite being a potent gonadotropin-releasing hormone analog, lacks a dopamine antagonist component. The inability of LHRHA to induce ovulation in this study may stem from the inhibitory effects of dopamine, which remain unmitigated. Additionally, the dosage of LHRHA administered in this study, although within the range recommended for other cyprinid species, might not have been sufficient to stimulate a reproductive response in Puntius sealei. Species-specific differences in hormone sensitivity and physiological requirements are well-documented, and this could explain the ineffectiveness of LHRHA under the current experimental conditions (de Abreu et al., 2022; Trudeau et al., 2010). Another factor that may have influenced the results is the role of environmental conditions, particularly water temperature. Spawning in cyprinids is often highly temperature-dependent, and the ambient temperatures observed during the study were within the typical range for tropical freshwater fish reproduction. However, subtle variations in temperature sensitivity between treatments could also influence hormone efficacy (Arantes et al., 2011; Benitez and Ovidio, 2018). Ovaprim, being a well-established hormone treatment, may exhibit greater consistency in triggering spawning across a wider range of environmental conditions compared to LHRHA (Acharjee et al., 2017; Yasmin et al., 2024).
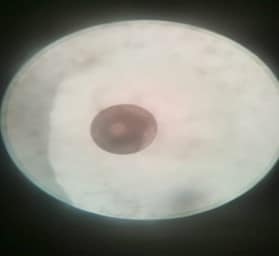 Figure 2. Unfertilized egg.
Figure 2. Unfertilized egg. 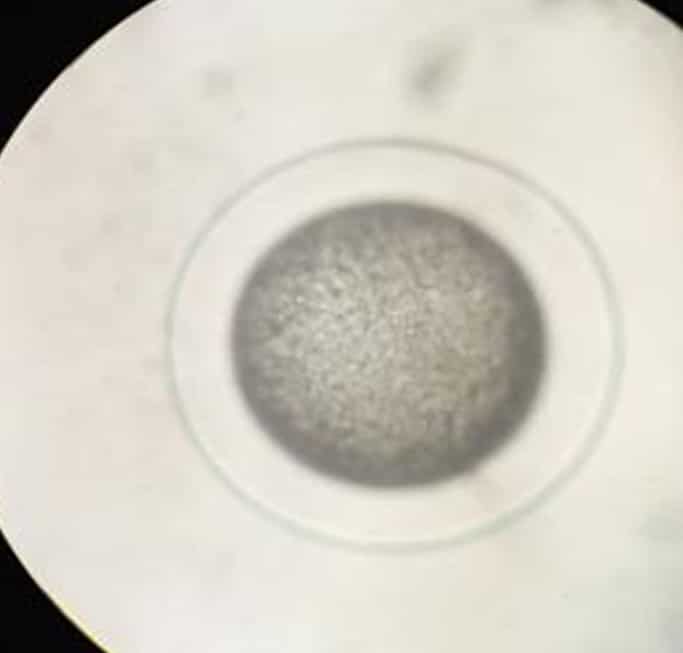 Figure 3. Fertilized egg 2 minutes after spawning.
Figure 3. Fertilized egg 2 minutes after spawning. 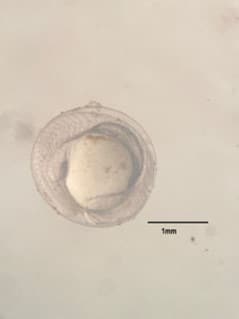 Figure 4. Organogenesis of developing embryo at 7 hours after fertilization.
Figure 4. Organogenesis of developing embryo at 7 hours after fertilization. 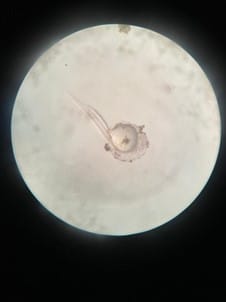 Figure 5. Prime stage showing emerging fish larvae at 20 hours after fertilization.
Figure 5. Prime stage showing emerging fish larvae at 20 hours after fertilization. 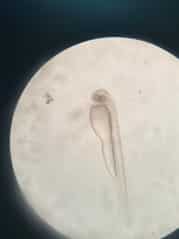 Figure 6. Fully developed larvae at 26 hours after fertilization.
Figure 6. Fully developed larvae at 26 hours after fertilization. The viability of fertilized eggs and successful embryonic development observed in the Ovaprim group further validate its reliability as an effective hormone treatment. The fertilized eggs underwent normal embryogenesis, with no evident abnormalities during key developmental stages, from cleavage to hatching. This suggests that Ovaprim does not compromise the quality of gametes or embryonic development, a critical consideration in artificial propagation programs (Khan et al., 2024; Kjørsvik et al., 2003). The findings of this study highlight the importance of hormone formulation and species-specific responses when selecting treatments for induced breeding. While Ovaprim has proven effective in this study, further investigations are necessary to refine protocols for the use of LHRHA in P. sealei. These studies could explore the addition of a dopamine antagonist, higher dosages, or the combination of LHRHA with other exogenous hormones to enhance its efficacy.
4. Conclusions
Ovaprim successfully induced spawning in Puntius sealei without causing any abnormalities during embryonic development, while LHRHA failed to trigger any spawning response. The lack of effectiveness of LHRHA could be attributed to factors such as insufficient dosage, the absence of a dopamine antagonist, or the need for additional exogenous hormones to enhance its efficacy. Further research is recommended to explore the optimal conditions and combinations of hormone treatments to improve reproductive outcomes and assess their impacts on the larvae of P. sealei
Acknowledgements
The authors would like to acknowledge the logistic and laboratory support of Universiti Malaysia Sarawak (UNIMAS) for conducting this study.
Data availability
All available data are presented in the article.
Informed consent statement
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contribution
Conceptualization: Lirong Yu Abit and Ahmad Syafiq Ahmad Nasir; Data collection: Lirong Yu Abit, Jongkar ak Grinang and Kamil Latif; Data analysis: Lirong Yu Abit, Jongkar ak Grinang and Kamil Latif; Figure preparation: Lirong Yu Abit. All authors critically reviewed the manuscript and agreed to submit final version of the manuscript. All authors critically reviewed the article and agreed to submit the final version of the article.



