The rise of antibiotic resistance and the emergence of new pathogenic strains present significant global health challenges, particularly in the 21st century. This crisis underscores the urgent need for alternative therapeutic strategies to combat zoonotic and opportunistic infections. In both developed and developing nations, there is a growing interest in herbal medicine as a safer and more accessible option for disease treatment and prevention (Islam et al., 2024 ![]() ). Approximately 85% of the global population relies on plant-based remedies, with demand increasing as modern pharmaceuticals face growing limitations, including adverse side effects and resistance development (Vaou et al., 2021
). Approximately 85% of the global population relies on plant-based remedies, with demand increasing as modern pharmaceuticals face growing limitations, including adverse side effects and resistance development (Vaou et al., 2021 ![]() ). In Africa, up to 80% of the population depends on traditional healers, and plant-based remedies play a crucial role in both human and veterinary health (Okaiyeto and Oguntibeju, 2021
). In Africa, up to 80% of the population depends on traditional healers, and plant-based remedies play a crucial role in both human and veterinary health (Okaiyeto and Oguntibeju, 2021 ![]() ; Yineger, 2008
; Yineger, 2008 ![]() ). Ethiopia, in particular, stands out, with 80% of the human population and over 90% of livestock reliant on plant-derived therapeutics (Abebe and Chane Teferi, 2021
). Ethiopia, in particular, stands out, with 80% of the human population and over 90% of livestock reliant on plant-derived therapeutics (Abebe and Chane Teferi, 2021 ![]() ).
).
The World Health Organization (WHO) estimates that between 35,000 and 70,000 plant species have been utilized as medicinal agents, representing a significant portion of the estimated 250,000 plant species globally (Saganuwan et al., 2016 ![]() ). However, only 17% of the world’s higher plant species have been scientifically investigated for their medicinal potential, leaving a vast, untapped resource for pharmaceutical development (Sen et al., 2015
). However, only 17% of the world’s higher plant species have been scientifically investigated for their medicinal potential, leaving a vast, untapped resource for pharmaceutical development (Sen et al., 2015 ![]() ). Ethiopia, one of Africa’s biodiversity hotspots, is home to around 6,500 species of flowering plants, including 92 families with known medicinal properties (UNEP, 2018). This biodiversity offers immense potential for the discovery of novel treatments, particularly in the face of multi-drug-resistaniceases, which currently cause around 700,000 deaths annually (Baynes, 2016
). Ethiopia, one of Africa’s biodiversity hotspots, is home to around 6,500 species of flowering plants, including 92 families with known medicinal properties (UNEP, 2018). This biodiversity offers immense potential for the discovery of novel treatments, particularly in the face of multi-drug-resistaniceases, which currently cause around 700,000 deaths annually (Baynes, 2016 ![]() ).
Despite significant advancements in modern medicine, the global health community has not introduced any new classes of antibiotics in the past three decades, with no promising candidates in sight (Chang et al., 2015
).
Despite significant advancements in modern medicine, the global health community has not introduced any new classes of antibiotics in the past three decades, with no promising candidates in sight (Chang et al., 2015 ![]() ). Consequently, attention has shifted towards traditional herbal remedies, which are increasingly viewed as safer alternatives due to their lower side effects (Balkrishna et al., 2024
). Consequently, attention has shifted towards traditional herbal remedies, which are increasingly viewed as safer alternatives due to their lower side effects (Balkrishna et al., 2024 ![]() ).
).
Among the most prominent medicinal plants, garlic (Allium sativum) has long been recognized for its antimicrobial properties. Numerous studies have confirmed garlic’s efficacy against a range of pathogens, including both gram-positive and gram-negative bacteria, as well as antibiotic-resistant strains (Gabriel et al., 2022 ![]() ; Magryś et al., 2021
; Magryś et al., 2021 ![]() ; Durairaj et al., 2020
; Durairaj et al., 2020 ![]() ). In Ethiopia, garlic is widely cultivated in the highland regions, including Adet, Ambo, Debre-work, Sinana, and Jimma. Similarly, black cumin seed (Nigella sativa), a plant renowned for its therapeutic properties, has been used for centuries across the Middle East, Eastern Europe, Asia, and Africa to treat various ailments (Hannan et al., 2021
). In Ethiopia, garlic is widely cultivated in the highland regions, including Adet, Ambo, Debre-work, Sinana, and Jimma. Similarly, black cumin seed (Nigella sativa), a plant renowned for its therapeutic properties, has been used for centuries across the Middle East, Eastern Europe, Asia, and Africa to treat various ailments (Hannan et al., 2021 ![]() ). In Ethiopia, black cumin seed is extensively grown in regions such as Amara, Oromiya, SNNP, and Gambella (Dessie et al., 2020
). In Ethiopia, black cumin seed is extensively grown in regions such as Amara, Oromiya, SNNP, and Gambella (Dessie et al., 2020 ![]() ). Honey, one of the oldest traditional medicines, has been valued for its therapeutic properties and has been documented in ancient medical literature for its antimicrobial and wound-healing activities (Petrosillo, 2008
). Honey, one of the oldest traditional medicines, has been valued for its therapeutic properties and has been documented in ancient medical literature for its antimicrobial and wound-healing activities (Petrosillo, 2008 ![]() ). The WHO reports a rising trend in the use of traditional medicine, particularly in developed countries, driven by the limitations of conventional medicine, the emergence of multi-drug-resistant pathogens, and the adverse effects of chemical drugs (WHO, 2014
). The WHO reports a rising trend in the use of traditional medicine, particularly in developed countries, driven by the limitations of conventional medicine, the emergence of multi-drug-resistant pathogens, and the adverse effects of chemical drugs (WHO, 2014 ![]() ).
).
Both Gram-positive and Gram-negative bacteria exhibit drug resistance. The World Health Organization released a list in 2017 that outlined the global priorities for antibiotic research and development. Among these, the most serious and urgent threats that call for increased international attention and quick action were highly resistant Enterobacteriaceae with Escherichia coli and Klebsiella pneumoniae (carbapenem-resistant and 3rd generation cephalosporin-resistant), as well as Pseudomonas aeruginosa (carbapenem-resistant) (Rana et al., 2024 ![]() ; Hossain et al., 2024
; Hossain et al., 2024 ![]() ; Julqarnain et al., 2022
; Julqarnain et al., 2022 ![]() ).
).
Given the growing threat of antimicrobial resistance and the limitations of current pharmaceuticals, there is an urgent need to explore and validate the medicinal properties of natural remedies scientifically. The novelty of this study lies in its comprehensive investigation of the synergistic antimicrobial effects of garlic, black cumin seed oil, and honey against specific zoonotic pathogens. Previous research has examined these natural agents individually, but few studies have explored their combined effects, particularly in the context of zoonotic bacteria and fungi. The objectives of this study are to extract and identify the bioactive compounds present in garlic and black cumin seed, compare the yields and antimicrobial activities of different extraction methods, and evaluate the antibacterial and antifungal efficacy of both single and combined extracts of garlic, black cumin seed, and honey against Staphylococcus aureus, Bacillus cereus, Escherichia coli, Salmonella typhi, and Candida albicans using the disk diffusion method. Ultimately, the study seeks to assess the potential of these natural substances as alternative treatments for bacterial and fungal infections.
By focusing on zoonotic pathogens, this research contributes not only to the field of antimicrobial resistance but also to the broader efforts in promoting the use of medicinal plants for sustainable healthcare solutions. The results of this study could pave the way for the development of novel, plant-based antimicrobial agents.
2. Materials and Methods
2.1 Ethical approval
No ethical approval is required for this study.
2.2 Study area
The study was conducted in in Jimma Town which is located 357 km away from Addis Ababa in southwest Ethiopia; and Gumay Woreda which is found in Jimma Zone of Oromia Regional State, and located at 415 km South West of Finfine and 69 km from Jimma town. Study was placed in Jimma University College of Agriculture and Veterinary Medicine School of Veterinary Medicine, Jimma, Ethiopia (Figure 1).
2.3 Processing of ingredients
2.3.1 Cold pressing of Nigella sativa oil
Nigella sativa seeds were collected, sieved to remove impurities, and processed using a cold press method at room temperature (25 °C). The oil was separated from the fibers overnight, filtered, and stored in amber glass bottles at room temperature. Ground Nigella sativa seeds were subjected to soxhlet extraction using acetone and ethanol as solvents. The extracted oils were concentrated using a rotary evaporator, weighed, and stored in amber glass bottles. Essential oils were extracted from ground Nigella sativa seeds using hydro-distillation. The process involved heating the seeds in distilled water, collecting the steam in a condenser, and separating the essential oil from the water. The oils were stored at 4 °C in amber glass bottles.
2.3.2 Preparation of experimental house
Garlic was crushed, dried for four weeks, and ground into a powder. The powder was macerated in ethanol and acetone, filtered, and the extracts were concentrated and stored in sterile containers at 4 °C.
2.3.3 Honey collection
Honey was collected from beekeepers in Gumay District and filtered through sterile gauze to ensure purity. Different concentrations of honey solutions (100%, 50%, and 25%) were prepared and stored at 4 °C.
2.3.4 Phytochemical screening
The garlic extracts were screened for various phytochemicals, including tannins, saponins, flavonoids, alkaloids, phenols, anthraquinones, and steroids, following established methods.
2.3.5 Microbial strains and culture preparation
Test microorganisms, including Staphylococcus aureus (ATCC 25923), Bacillus cereus (ATCC 14579), Escherichia coli (ATCC 25922), Salmonella typhi(ATCC 19430), and Candida albicans (ATCC 10237), were obtained from the Biology Department of Jimma University. The organisms were sub-cultured on nutrient agar, standardized, and used for subsequent antimicrobial assays.
2.3.6 Antimicrobial sensitivity (disc diffusion method)
The antimicrobial activity of the extracts was assessed using the disc diffusion method. Sterile discs were impregnated with plant extracts and placed on inoculated agar plates. The zones of inhibition were measured after 24 hours of incubation at 37 °C.
2.3.7 Minimum inhibitory concentration (MIC)
TThe MIC is the lowest concentration of an extract that inhibits visible bacterial growth (Radojevic et al., 2012). For this study, stock solutions of 50 mg/ml of each extract were prepared, and double dilutions were performed to achieve concentrations of 25 mg/ml, 12.5 mg/ml, and 6.25 mg/ml (CLSI, 2012 ![]() ). Each dilution was mixed with 10 ml of Muller Hinton broth and inoculated with 20 µl of a bacterial suspension adjusted to 0.5 McFarland standards. After incubation at 37 °C for 24 hours, the mixtures were further inoculated on agar plates. The MIC was recorded as the lowest concentration that inhibited bacterial growth. Positive and negative controls were used, and all tests were performed in triplicate.
). Each dilution was mixed with 10 ml of Muller Hinton broth and inoculated with 20 µl of a bacterial suspension adjusted to 0.5 McFarland standards. After incubation at 37 °C for 24 hours, the mixtures were further inoculated on agar plates. The MIC was recorded as the lowest concentration that inhibited bacterial growth. Positive and negative controls were used, and all tests were performed in triplicate.
2.4 Data analysis
Data were recorded in Microsoft Excel and analyzed using SPSS 20. Antimicrobial activity was expressed as mean inhibition zones for the different extracts tested against each bacterial strain. The data were expressed as mean of antimicrobial activities of the extracts namely: GAE, GEE, BAE, BEE, and HONEY. For each, we were calculated as the mean comparison value of the zone of inhibition obtained against all individual bacteria.
3. Results
3.1 Percentage yield of extract with different method and with different solvent
Black cumin extracted with the soxhlet solvent extraction method yielded 23 g and 19 g extracts from a 63 g sample/300 ml of ethanol and acetone solvent extracted, respectively. Black cumin extracted with cold pressing extraction method yielded 12 g extracts from 100 g sample. Black cumin extracted with hydro distillation extraction method yielded 1 g extracts from 100g sample /1000 ml of water. Garlic extracted with cold maceration extraction method yielded 6g and 3.7 g extracts from 100 g sample/500 ml of ethanol and acetone solvent extracted respectively (Table 1).
Table 1. Results of extracted garlic and black cumin via different method and different solvent.
3.2 Phytochemical analysis
The phytochemical screening of the samples revealed the presence of several bioactive compounds. Tannins were detected in the acetone extract with a discoloration reaction using bromine water. Saponins produced a frothy reaction in the ethanol extract but were absent in the acetone extract. Flavonoids were present in both ethanol and acetone extracts, indicated by a yellow color change with HCl. Phlobatannins, phenols, glycosides, and steroids were also present in both extracts, showing red precipitation, dirty green, reddish-brown, and green bluish colors respectively. Alkaloids were detected in the ethanol extract with an orange-red color change but were absent in the acetone extract. However, anthraquinones and terpenoids were not detected in either extract, as indicated by the absence of pink coloration and red-violet color, respectively (Table 2).
Table 2. Phytochemical screening.
3.3 Antimicrobial sensitivity test
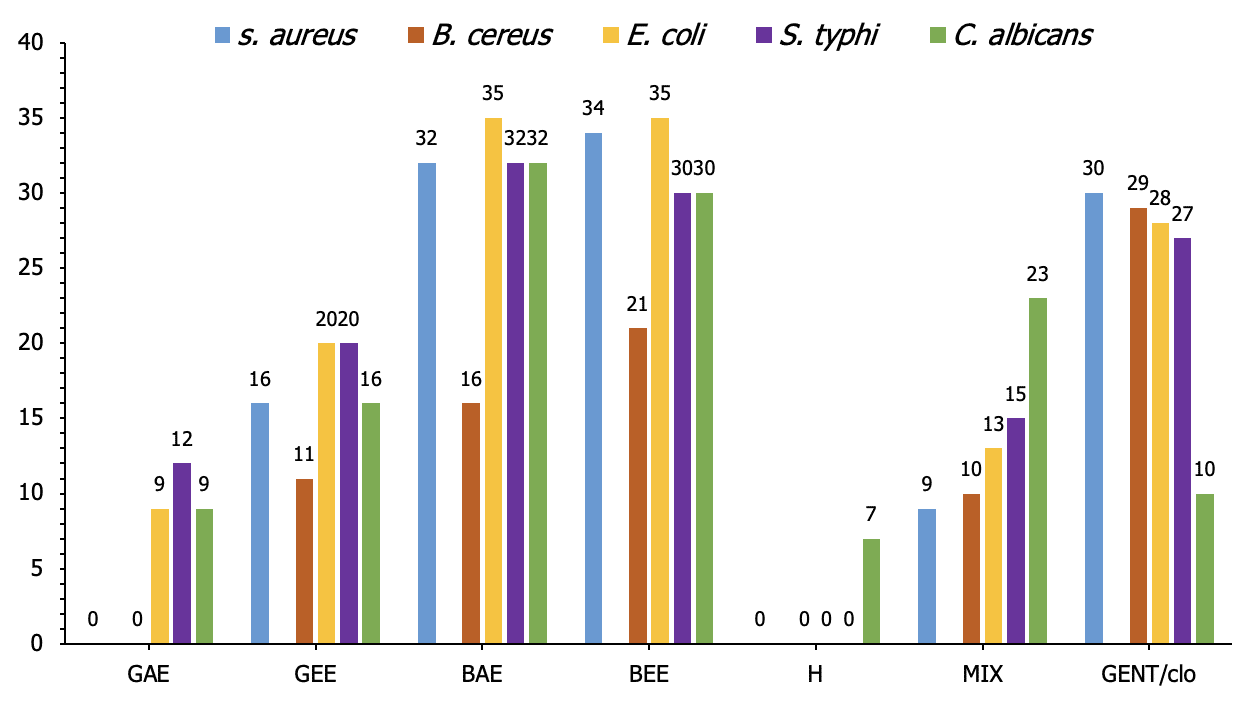
The antimicrobial activity (measured in mm) of 50% concentrations of black cumin oil, garlic, and honey against the tested strains revealed varying levels of inhibition. Black Cumin Ethanol Extract (BEE) exhibited the most potent activity, especially against E. coli and S. aureus, with inhibition zones of 35 mm and 34 mm, respectively. Garlic Ethanol Extract (GEE) also showed significant antimicrobial effects, with inhibition zones ranging from 11 mm against B. cereus to 20 mm against both E. coli and S. typhi. Garlic Acetone Extract (GAE) displayed the least antimicrobial activity, with inhibition zones ranging from 0 mm against S. aureus and B. cereus to 12 mm against S. typhi. Honey demonstrated limited activity, with a 7 mm inhibition zone only against C. albicans. The mixture of the extracts provided moderate inhibition across all tested strains, while the control group (Gentamycin/Clotrimazole) consistently showed substantial inhibition, with zones ranging from 10 mm against C. albicans to 30 mm against S. aureus (Figure 2).
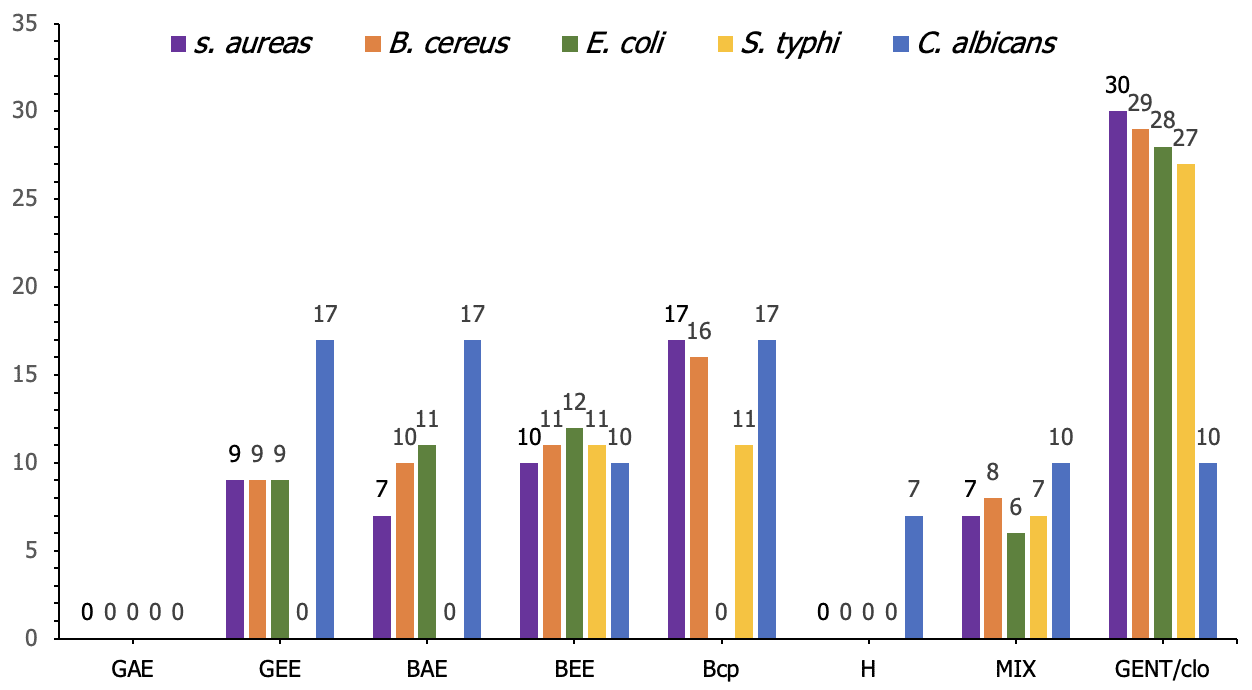
The antimicrobial activity (measured in mm) of 25% concentrations of black cumin oil, garlic, and honey against the tested strains demonstrated varying degrees of inhibition. Black Cumin Ethanol Extract (BEE) showed potency against E. coli and S. aureus, with inhibition zones of 12 mm and 10 mm, respectively. Garlic Ethanol Extract (GEE) also exhibited notable antimicrobial effects, with inhibition zones 17 mm against C. albicans and 9 mm against S. aureus, B. cereus and E. coli. In contrast, Garlic Acetone Extract (GAE) and Honey displayed no antimicrobial activity. The mixture of the extracts offered moderate inhibition across all tested strains, while the control group (Gentamycin/Clotrimazole) consistently showed strong inhibition (Figure 3).
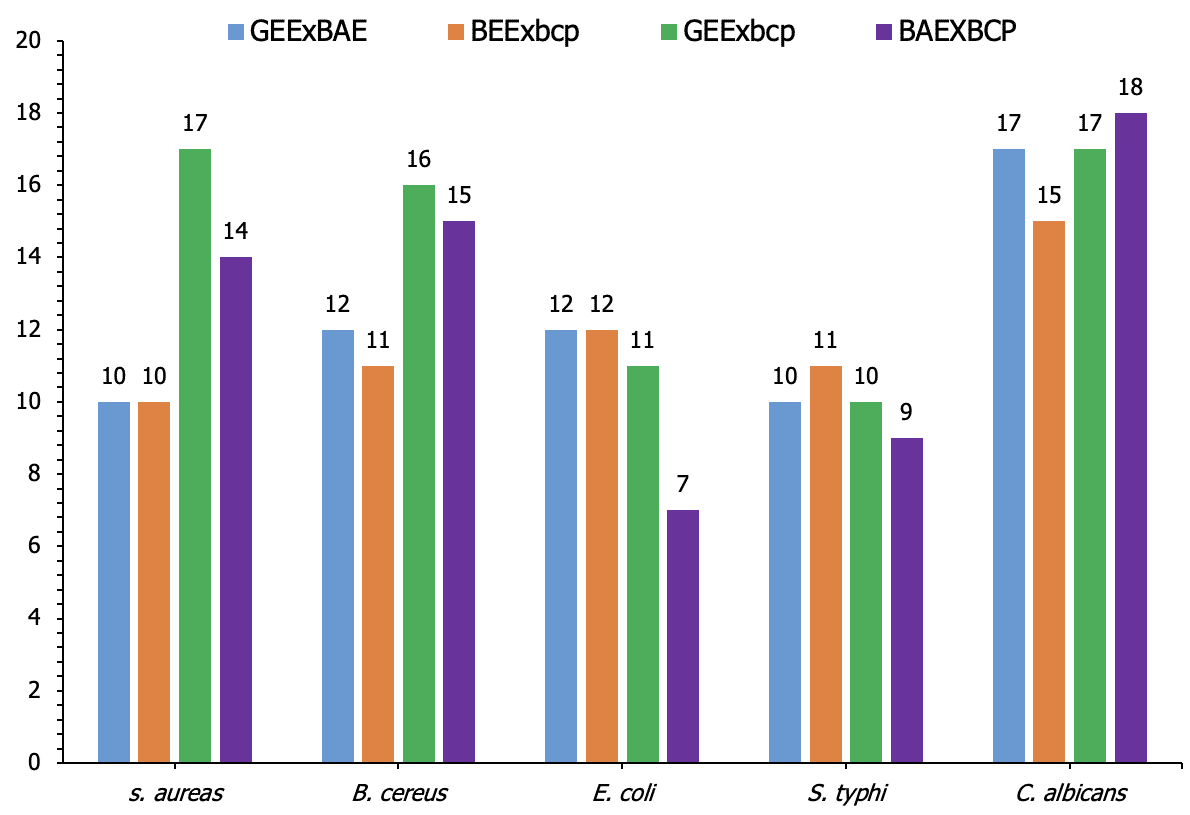
The results presented in Table 5 illustrate the synergistic antimicrobial effects of various combinations of garlic ethanol extract (GEE), black cumin acetone extract (BAE), and black cumin oil extract (BCP). Among the tested pathogens, Staphylococcus aureus showed the highest inhibition zone of 17 mm when treated with a mixture of GEE and BCP (GEExBCP), followed by 14 mm with BAExBCP. Similarly, Bacillus cereus exhibited the greatest inhibition zone of 16 mm with the GEExBCP combination, while the other combinations demonstrated slightly lower inhibition zones, ranging from 11 to 15 mm. For Escherichia coli, the GEE and BAE combinations (GEExBAE and BEExBCP) both resulted in inhibition zones of 12 mm, whereas the GEExBCP combination showed a slightly reduced effect of 11 mm. In contrast, Salmonella typhi displayed relatively consistent inhibition zones across all combinations, with values ranging from 9 to 11 mm. The highest inhibition was observed with BEExBCP at 11 mm. Candida albicans demonstrated potent inhibition, with the highest zone of 18 mm for the BAExBCP combination, followed by 17 mm for both GEExBAE and GEExBCP. These results indicate that certain combinations, particularly GEExBCP and BAExBCP, exhibit enhanced antimicrobial activity, potentially due to the synergistic effects of the extracts (Figure 4).
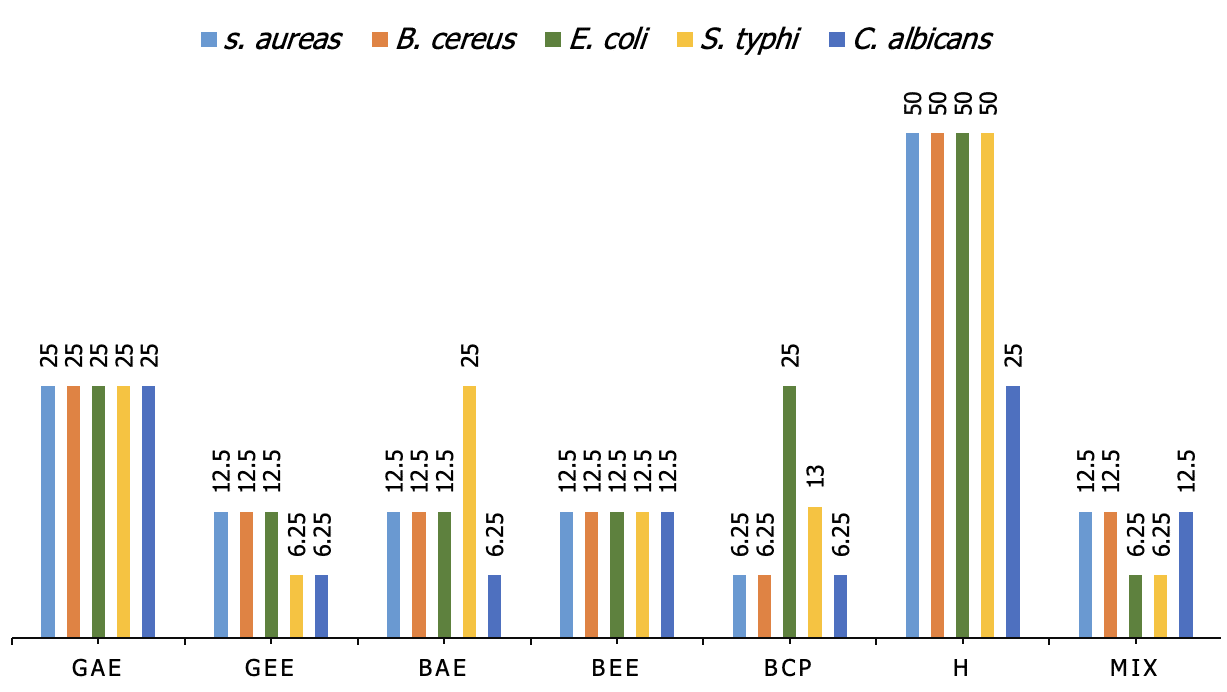
The minimum inhibitory concentration (MIC) results, reveal variations in antimicrobial efficacy across different extracts and pathogens. For Staphylococcus aureus and Bacillus cereus, black cumin oil (Bcp) demonstrated the lowest MIC at 6.25%, while the other extracts, including garlic acetone extract (GAE), garlic ethanol extract (GEE), black cumin ethanol extract (BEE), and black cumin acetone extract (BAE), had MIC values of 12.5%, except for GAE at 25%. Similarly, for Escherichia coli, the mixture of extracts exhibited the lowest MIC at 6.25%, with Bcp and other extracts showing MIC values ranging from 12.5% to 25%. In the case of Salmonella typhi, the most potent inhibition was observed for GEE and the mixture at 6.25%, while Bcp had a MIC of 13%. Finally, for Candida albicans, GEE, BAE, and Bcp demonstrated the most potent activity with a MIC of 6.25%, while other extracts showed higher MICs, including honey (H) at 25%. These findings highlight the superior antimicrobial activity of Bcp and the synergistic mixture of extracts (Figure 5).
4. Discussion
The extraction efficiency and antimicrobial efficacy of plant-derived compounds are heavily influenced by the extraction method and the solvent used. This study evaluated the yields and antimicrobial activities of garlic and black cumin seed oil extracts, obtained through various extraction techniques, including cold pressing, Soxhlet extraction, hydrodistillation, and cold maceration, using different solvents.
The yield from cold pressing of black cumin seed (12 g from 100 g) was moderate compared to Soxhlet extraction and hydrodistillation. This result aligns with Benkeblia (2004 ![]() ), who reported that the low heat generated during cold pressing leads to medium yields and moderate antimicrobial activity. The Soxhlet extraction, especially with ethanol, produced the highest yield (23 g from 63 g), likely due to ethanol's greater polarity, which makes it more efficient at extracting bioactive compounds than acetone. This is consistent with findings Benkaci et al. (2012
), who reported that the low heat generated during cold pressing leads to medium yields and moderate antimicrobial activity. The Soxhlet extraction, especially with ethanol, produced the highest yield (23 g from 63 g), likely due to ethanol's greater polarity, which makes it more efficient at extracting bioactive compounds than acetone. This is consistent with findings Benkaci et al. (2012 ![]() ), who reported that black cumin seed oil extraction with ethanol yields higher percentages due to better solubility of the seed's chemical content.
), who reported that black cumin seed oil extraction with ethanol yields higher percentages due to better solubility of the seed's chemical content.
Hydrodistillation, however, yielded only 1% essential oil from black cumin seed, much lower than the other methods (soxhlet extraction and cold pressing). The challenges of oil recovery during hydrodistillation, such as oil escaping with water, contributed to its inefficiency, which contrasts with some studies suggesting that higher temperatures can improve oil yields. This finding is supported by Benkeblia (2004 ![]() ), who noted that low heat during distillation often results in medium or low yields.
), who noted that low heat during distillation often results in medium or low yields.
Garlic extraction using cold maceration with ethanol also outperformed acetone, producing 6 g of extract compared to 3.7 g with acetone. Ethanol’s higher polarity likely contributed to the higher yield, a conclusion supported by Wolde et al. (2018 ![]() ), who reported similar results with more polar solvents. The qualitative phytochemical analysis revealed the presence of several bioactive compounds, such as phenols, saponins, steroids, and flavonoids, in the ethanol extracts, which were either absent or less abundant in the acetone extracts. This is consistent with the findings of Jadon and Dixit (2014
), who reported similar results with more polar solvents. The qualitative phytochemical analysis revealed the presence of several bioactive compounds, such as phenols, saponins, steroids, and flavonoids, in the ethanol extracts, which were either absent or less abundant in the acetone extracts. This is consistent with the findings of Jadon and Dixit (2014 ![]() ), who also noted similar phytochemical profiles in Allium species, highlighting their therapeutic potential.
), who also noted similar phytochemical profiles in Allium species, highlighting their therapeutic potential.
The antimicrobial activity results further demonstrated the effectiveness of ethanol as an extraction solvent. Ethanol-based garlic extracts exhibited the strongest inhibition zones against E. coli and S. typhi at 50 mg/ml concentrations, with inhibition zones of 22 mm and 20 mm, respectively. In contrast, the acetone extracts showed lower antimicrobial activity, with maximum inhibition zones of 12 mm against S. typhi. The superior antimicrobial performance of ethanol extracts can be attributed to their higher concentration of polar bioactive compounds, such as sulfur compounds and phenolics, which are known for their antimicrobial properties (Cushnie and Lamb, 2020 ![]() ).
).
Black cumin seed oil extracted with ethanol displayed the highest antimicrobial activity, with inhibition zones of 35 mm against E. coli and 34 mm against S. aureus, outperforming the positive control (gentamycin). Its antifungal activity was also notable, with a 32 mm inhibition zone against Candida albicans, compared to 10 mm for clotrimazole, the positive control. These findings are in line with Yimer (2019), who also documented strong antimicrobial properties in black cumin seed oil.
When comparing the overall efficacy, black cumin seed oil extracted with ethanol and acetone was more potent than garlic extracts, and significantly more effective than extracts obtained via hydrodistillation. The combination of garlic extract, black cumin seed oil, and honey exhibited moderate synergistic effects, though the individual extracts, particularly black cumin seed oil, were more effective. This observation is supported by Ncube et al. (2012 ![]() ) and Zhou et al. (2016
) and Zhou et al. (2016 ![]() ), who emphasized the importance of synergistic interactions in enhancing the efficacy of herbal remedies.
), who emphasized the importance of synergistic interactions in enhancing the efficacy of herbal remedies.
The minimum inhibitory concentration (MIC) results further demonstrated the potency of these extracts. For instance, black cumin seed oil extracted with ethanol had an MIC of 12.5%, while garlic acetone extracts showed a higher MIC of 25%, indicating lower potency. These findings are consistent with previous studies, such as Hutabarat (2021 ![]() ) and Sadeghian and Ghazvini (2002
) and Sadeghian and Ghazvini (2002 ![]() ), which highlighted the superior efficacy of ethanol extracts in inhibiting bacterial growth.
), which highlighted the superior efficacy of ethanol extracts in inhibiting bacterial growth.
While this study provides valuable insights into the antimicrobial potential of garlic, black cumin seed oil, and honey, there are several limitations. First, the study was conducted entirely in vitro, which may not fully represent how these extracts interact in living organisms. The lack of in vivo testing limits the ability to translate these findings into practical therapeutic applications. Second, the exact mechanisms of action of the bioactive compounds were not explored, leaving questions about how these compounds exert their antimicrobial effects. Third, the study focused on a limited number of zoonotic pathogens, and broader testing on additional microbial species would be necessary to confirm the generalizability of the results. Lastly, while the study demonstrated the efficacy of these natural products, potential variations in extract composition due to factors like plant origin and environmental conditions were not considered, which could affect reproducibility.
The study conclusively demonstrates the significant antimicrobial properties of garlic, black cumin seed oil, and honey, individually and in combination, against zoonotic pathogens. The findings underscore the potential of these natural products as alternative treatments to combat bacterial and fungal infections, particularly in light of rising antimicrobial resistance. Despite the promising in vitro results, further in vivo studies are required to validate these findings in practical, real-world applications. Additionally, understanding the mechanisms behind the antimicrobial actions of these extracts will be crucial for developing them into reliable therapeutic agents.
5. Conclusions
The study conclusively demonstrates the significant antimicrobial properties of garlic, black cumin seed oil, and honey, whether used individually or in combination, against various foodborne, opportunistic, and zoonotic infectious strains. The findings underscore the potential of these natural products as effective food additives and supplements, providing both nutritional and medicinal benefits. The study also highlights that solvent extraction, particularly with ethanol, is superior to non-solvent methods in enhancing antimicrobial activity. While the results are promising, further in vivo evaluations are necessary to bridge the gap between laboratory findings and real-world applications. The study's outcomes suggest that these natural remedies hold promise for developing new antimicrobial drugs, emphasizing the need for continued research, drug formulation testing, and policy development for the conservation and utilization of medicinal plants.
Acknowledgements
The author would like acknowledge to the Jimma University College of Agriculture and Veterinary Medicine School of Veterinary Medicine, Jimma, Ethiopia.
Data availability statement
All relevant data are within the manuscript.
Informed consent statement
No informed consent was required to conduct the study.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Conceptualization: Hafiz Esmael; Methodology: Hafiz Esmael and Abdu Mohamed; Formal analysis: Hafiz Esmael; Original draft preparation: Hafiz Esmael and Kazi Abdus Sobur; Manuscript formatting and result interpretation: Md. Zaminur Rahman and Md. Mosharraf Hossen; First draft development, data analysis, review and editing: Kazi Abdus Sobur and Md. Mosharraf Hossen; Easing it, final review and editing the manuscript: Md. Ashiquen Nobi.



