Mitochondrial Thioredoxin-Interacting Protein (TXNIP) has emerged as a crucial regulator of cellular redox homeostasis, significantly influencing oxidative stress responses, apoptosis, and metabolic regulation. Initially identified as a negative regulator of thioredoxin (TRX), TXNIP plays a pivotal role in modulating thioredoxin (TRX) activity (Nishiyama et al., 1999). The discovery of TXNIP’s localization in mitochondria further expanded its functional landscape, linking it to critical cellular processes such as reactive oxygen species (ROS) regulation, mitochondrial integrity, and cell death pathways (Dutta et al., 2005; Saxena et al., 2010).
Mitochondria serve as the primary site for ROS production, generating approximately 90% of cellular ROS (Balaban et al., 2005). The thioredoxin system, particularly mitochondrial TRX2, acts as a major antioxidant defense mechanism to counteract oxidative stress. TXNIP inhibits TRX2 activity, leading to increased ROS accumulation, mitochondrial dysfunction, and activation of apoptotic pathways (Saxena et al., 2010). This dysregulation of redox balance has been implicated in several pathological conditions, including myocardial infarction, diabetes, neurodegeneration, and nephrotic syndrome (Nakayama et al., 2021; Park et al., 2022).
Beyond apoptosis, TXNIP has been linked to ferroptosis, an iron- dependent form of cell death driven by lipid peroxidation (Dixon et al., 2012). Studies have demonstrated that TXNIP modulates mitochondrial labile iron levels and ROS, thereby influencing ferroptotic pathways in various disease contexts (Karmi et al., 2021; Chen et al., 2024). Furthermore, TXNIP plays a vital role in mitophagy, a selective process that removes damaged mitochondria to maintain cellular homeostasis. Its dysregulation has been associated with retinal disorders such as diabetic retinopathy (Devi et al., 2017).
Another significant aspect of TXNIP’s function is its involvement in inflammasome activation. TXNIP directly interacts with the NLRP3 inflammasome in response to mitochondrial ROS, leading to inflammatory cascades that exacerbate disease progression in conditions such as myocardial infarction and kidney injury (Zhou et al., 2010, 2011; Schroder and Tschopp, 2010).
Despite substantial advances in understanding mitochondrial TXNIP, the precise molecular mechanisms underlying its pathological roles remain incompletely understood. This review aims to consolidate current knowledge on mitochondrial TXNIP, highlighting its regulatory mechanisms, physiological significance, and implications in various diseases. Understanding TXNIP’s role in mitochondrial dysfunction and redox imbalance may pave the way for novel therapeutic strategies targeting oxidative stress-related disorders.
2. The discovery of mitochondrial localization of TXNIP
The first localization of Thioredoxin-Binding Protein-2 (TBP-2), also known as TXNIP, in the mitochondria was reported by Dutta et al. (2005). This groundbreaking research provided evidence of TBP-2's mitochondrial presence, highlighting its potential role in regulating mitochondrial redox balance through its interaction with mitochondrial thioredoxin (TRX2). The study linked TBP-2's mitochondrial localization to its involvement in oxidative stress responses and carcinogenesis. It also identified the mechanisms underlying TBP-2 loss, including epigenetic silencing and proteasomal degradation, emphasizing its importance in maintaining mitochondrial integrity and preventing oxidative stress-driven tumorigenesis. TXNIP's intracellular shuttling and its critical role in mitochondrial function, maintenance of cellular redox balance and metabolic regulation were further explored and highlighted by Saxena et al. (2010).
3. Functional implications of mitochondrial TXNIP
Using a yeast two hybrid system, TXNIP (TBP-2) was identified as a binding partner of TRX (Nishiyama et al., 1999). They observed this association both in vitro and in vivo. They reported TXNIP (TBP-2) as a negative regulator of biological function of TRX. The discovery of physical interaction between TXNIP and mitochondrial thioredoxin (TRX2) has profound implications for understanding cellular redox homeostasis and its dysregulation in pathological conditions (Dutta et al., 2005). About 90% of the cellular ROS are produced inside the mitochondria (Balaban et al., 2005). The thioredoxin system, particularly mitochondrial thioredoxin (TRX2), is crucial for mitigating oxidative damage. TXNIP’s interaction with TRX2 in the mitochondria suggests it acts as a critical regulator, modulating ROS levels and influencing mitochondrial function.
3.1 Regulation of oxidative stress
By inhibiting TRX2 activity, mitochondrial TXNIP promotes the accumulation of ROS. While this regulation is essential for signaling and apoptosis under physiological conditions, excessive TXNIP activity or its dysregulation can lead to oxidative stress and mitochondrial damage. This mechanism is particularly relevant in diseases characterized by oxidative stress, such as neurodegenerative disorders, cardiovascular diseases, and cancer (Figure 1).
 Figure 1. Mitochondrial presence of TXNIP causes excess accumulation of ROS which subsequently drives multiple events including cell death.
Figure 1. Mitochondrial presence of TXNIP causes excess accumulation of ROS which subsequently drives multiple events including cell death.
3.2 Role in apoptosis
The mammalian thioredoxin 2 (TRX-2) gene was first time cloned and its product was shown to localize in the mitochondria by Spyrou et al. (1997). It was demonstrated using TRX-2-deficient chicken B-cell lines (DT40) with a tetracycline-repressible TRX-2 transgene that suppression of TRX-2 expression led to increased ROS levels, decreased mitochondrial and cellular glutathione (GSH), and activation of the intrinsic apoptotic pathway (Tanaka et al., 2002). Experimental approaches included flow cytometry to measure intracellular ROS and caspase activity, western blotting to assess cytochrome c release, and co-immunoprecipitation assays to confirm the direct interaction between TRX-2 and cytochrome c. The results revealed that TRX-2 deficiency triggers cytochrome c release from mitochondria, activates caspase-9 and caspase-3, and promotes apoptosis without significant mitochondrial membrane depolarization. These findings highlight TRX-2’s dual role in scavenging ROS and regulating the mitochondrial apoptotic signaling pathway.
TRX-2 overexpressing human embryonic kidney (HEK-293) cells also demonstrated increased resistance to etoposide-induced apoptosis while simultaneously heightening sensitivity to rotenone, a complex I inhibitor (Damdimopoulos et al., 2002). This highlights its dual role in cell survival and vulnerability under different stress conditions. TRX-2's anti-apoptotic effects are attributed to its ability to maintain mitochondrial integrity and scavenge reactive oxygen species (ROS), reducing oxidative damage. Conversely, its involvement in enhancing suggests a regulatory interaction with ATP synthase activity, as confirmed through treatments with mitochondrial inhibitors like oligomycin.
TXNIP is an endogenous inhibitor of anti-oxidant property of mitochondrial TRX-2. Under normal conditions, TXNIP is mainly localized in the nucleus, but oxidative stress triggers its translocation to the cytosol and mitochondria. In the cytosol, TXNIP binds to thioredoxin-1 (TRX1), releasing apoptosis signal-regulating kinase 1 (ASK1) from its inhibitory complex with TRX1. This activates the p38 mitogen-activated protein kinase (p38 MAPK) pathway, promoting apoptosis (Fujino et al., 2007; Lv et al., 2011; Lu et al., 2012).
In mitochondria, TXNIP interacts with mitochondrial thioredoxin-2 (TRX2), disrupting its antioxidant function. This interaction causes ASK1 activation and phosphorylation, leading to the release of cytochrome c (Cyto c) from mitochondria into the cytosol (Saxena etal., 2010; Lu et al., 2012). Cyto c activates apoptotic protease-activating factor-1 (Apaf-1), which forms the apoptosome, subsequently activating caspase-9 and caspase-3. These events drive programmed cell death (Würstle et al., 2012; Yoshihara et al., 2014; Nasoohi et al., 2018).
In pancreatic β-cells, which are highly sensitive to oxidative stress due to low antioxidant enzyme levels, TXNIP overexpression triggers apoptosis through this mitochondrial pathway. Conversely, TXNIP depletion enhances β-cell survival under stress by improving protective signaling pathways like AKT/Bcl-xL (Minn et al., 2005; Chen et al., 2008). TXNIP mediated apoptosis has also been implicated in various disorders, such as diabetes and nephrotic syndrome, making it a potential therapeutic target for conditions linked to oxidative stress-induced cell death.
3.3 Role in ferroptosis
Ferroptosis, a regulated form of cell death driven by iron-dependent lipid peroxidation, is tightly associated with mitochondrial dysfunction and oxidative stress. Ferroptosis was first observed in 2003 and the name “ferroptosis” was coined in 2012 (Dolma et al., 2003; Yang and Stockwell, 2008; Dixon et al., 2012). Recent studies highlight TXNIP's contribution to the mitochondrial pathway of ferroptosis. An important link was reported among CISD2 (NAF-1) protein, tumor suppressor TXNIP expression, mitochondrial labile iron (mLI), mitochondrial ROS and ferroptosis (Karmi et al., 2021). Disruption of CISD2 function resulted in the enhanced expression of TXNIP that was dependent on the accumulation of mitochondrial labile iron (mLI) and associated with ferroptosis activation. The combined effects of dexmedetomidine and argon on ferroptosis in donation after circulatory death (DCD) porcine livers was investigated (Chen et al., 2024). Their findings suggest that this combination therapy mitigates ferroptosis by targeting TXNIP-mediated oxidative stress. By reducing TXNIP expression or activity, the antioxidant function of TRX is preserved, leading to decreased ROS levels and protection against ferroptosis.
3.4 Role in mitophagy
Mitochondrial TXNIP has emerged as a crucial regulator of mitophagy, the selective autophagic degradation of damaged mitochondria. Proper mitophagy ensures mitochondrial quality control, preventing the accumulation of dysfunctional mitochondria that contribute to oxidative stress and metabolic dysregulation. In retinal Müller cells, which are essential for maintaining retinal structure and function, high-glucose conditions can induce oxidative stress, leading to mitochondrial damage.
Under such conditions, TXNIP expression is upregulated, resulting in the inhibition of thioredoxin's antioxidant activity and an increase in reactive oxygen species (ROS). Elevated ROS levels can damage mitochondria, triggering mitophagy as a protective mechanism to remove dysfunctional mitochondria and maintain cellular homeostasis.
Under high-glucose conditions, TXNIP was found to regulate mitophagy in retinal Müller cells (Devi et al., 2017). Their findings suggest that increased TXNIP expression leads to enhanced mitophagic activity, which may serve as a compensatory response to mitigate mitochondrial damage induced by oxidative stress. However, prolonged high-glucose exposure and sustained TXNIP upregulation can overwhelm the mitophagic machinery, potentially contributing to mitochondrial dysfunction and the progression of diabetic retinopathy.
3.5 Role in inflammasome assembly and activation
The TXNIP is a critical regulator of oxidative stress and inflammation. Its translocation to mitochondria plays a pivotal role in the assembly and activation of the NLRP3 inflammasome, a key component of the innate immune response. This mechanism has been elaborated in several landmark studies. Mitochondria play an integral role to NLRP3 inflammasome activation by providing reactive oxygen species (ROS) and promoting the release of mitochondrial DNA into the cytosol, which serves as a ligand for inflammasome assembly (Zhou et al., 2011). This study emphasized that mitochondrial dysfunction is closely tied to inflammasome activity and the ensuing inflammatory responses (Figure 2).
Mitochondrial signals were found as critical requirement in recruiting and activating NLRP3 prior inflammasome formation (Schroder and Tschopp, 2010). This study provided a comprehensive overview of the inflammasome pathways and underlined the interplay between cellular stress and inflammatory responses.
Direct physical binding of TXNIP and NLRP3 takes place before the assembly and activation of NLRP3 inflammasome (Zhou et al., 2010). This binding is facilitated by TXNIP's translocation to mitochondria, where it senses mitochondrial ROS, thus acting as a bridge between oxidative stress and inflammasome activation.
Additionally, it was recently identified that blocking CHOP-dependent TXNIP translocation to mitochondria can attenuate NLRP3 inflammasome activation (Park et al., 2022). This study highlighted a therapeutic avenue by demonstrating that inhibiting TXNIP shuttling to mitochondria reduces mitochondrial dysfunction, inflammasome activation, and related inflammatory damage in nephrotic syndrome.
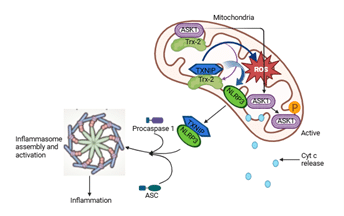 Figure 2. Mitochondrial TXNIP driven excess accumulation of mtROS, mtDNA and other mtDAMPs induce inflammasome complex assembly and activation.
Figure 2. Mitochondrial TXNIP driven excess accumulation of mtROS, mtDNA and other mtDAMPs induce inflammasome complex assembly and activation. Together, these studies underscore the importance of TXNIP's mitochondrial localization in bridging oxidative stress and the activation of the NLRP3 inflammasome. Targeting this pathway holds potential for mitigating inflammation-driven diseases, providing valuable insights into the therapeutic regulation of inflammasomes (Table 1).
Table 1. Mitochondrial TXNIP functions in oxidative stress, cell death, and inflammation.
4. Pathophysiological implications of mitochondrial TXNIP/TBP-2
Mitochondrial TXNIP dysregulation, primarily through inhibiting TRX2 and increasing ROS, leads to oxidative stress, apoptosis, and inflammation across various pathologies. In myocardial infarction, this exacerbates injury; in diabetes, it contributes to beta-cell dysfunction; in nephrotic syndrome, it damages kidney cells. However, in retinitis pigmentosa, controlled TXNIP overexpression can paradoxically enhance antioxidant responses and preserve vision.
4.1 Implications in myocardial infraction
Mitochondrial TXNIP has emerged as a critical regulator in the pathophysiology of myocardial infarction (MI), particularly due to its influence on oxidative stress, apoptosis, and inflammatory responses. TXNIP is known to bind to mitochondrial thioredoxin (TRX2), inhibiting its antioxidant activity and exacerbating mitochondrial reactive oxygen species (ROS) production. This dysregulation contributes to oxidative damage, a central mechanism in myocardial ischemia-reperfusion (I/R) injury, a hallmark of MI.
A significant insights into the role of mitochondrial TXNIP in MI was provided using a mouse model with a TXNIP C247S mutation (Nakayama et al., 2021). This mutation disrupts TXNIP's ability to bind to TRX2, effectively mitigating its pro-oxidative effects. The study demonstrated that mice harboring the TXNIP C247S mutation exhibited reduced myocardial injury following acute MI, as evidenced by smaller infarct sizes, improved cardiac function, and decreased levels of oxidative stress markers. This protective phenotype highlights the importance of TXNIP-TRX2 interactions in regulating mitochondrial redox homeostasis during MI.
Furthermore, the study linked the TXNIP C247S mutation to attenuated apoptosis and inflammatory responses in the heart. Reduced cytochrome c release and caspase activation were observed, indicating that the mutation protects against mitochondrial-mediated apoptosis. Additionally, the mutation diminished NLRP3 inflammasome activation, leading to lower levels of pro-inflammatory cytokines such as IL-1β and IL-18. These findings underscore the dual role of mitochondrial TXNIP in promoting both apoptosis and inflammation, processes that exacerbate myocardial injury during MI.
The work by Nakayama et al. (2021) provides a compelling rationale for targeting mitochondrial TXNIP as a therapeutic strategy in MI. By preventing TXNIP-TRX2 interactions, it may be possible to preserve mitochondrial integrity, reduce oxidative stress, and mitigate downstream apoptotic and inflammatory cascades. These findings pave the way for the development of TXNIP-targeted therapies aimed at improving outcomes in patients with acute MI.
4.2 mplications in diabetes
Mitochondrial TXNIP plays a critical role in the pathophysiology of diabetes by modulating oxidative stress, inflammation, and mitochondrial function. TXNIP negatively regulates thioredoxin, a vital antioxidant protein, leading to increased reactive oxygen species (ROS) production and oxidative stress. In the context of diabetes, elevated glucose levels induce TXNIP expression, contributing to mitochondrial dysfunction and beta-cell apoptosis. This dysregulation impairs insulin secretion and exacerbates insulin resistance, hallmarks of both type 1 and type 2 diabetes. Additionally, TXNIP influences mitochondrial metabolic processes and inflammasome activation, promoting inflammatory responses that further aggravate diabetic complications. It was demonstrated that under oxidative stress, TXNIP translocates from the nucleus to mitochondria in pancreatic beta cells, where it binds to mitochondrial thioredoxin 2 (Trx2). This interaction leads to the activation of apoptosis signal-regulating kinase 1 (ASK1), triggering the mitochondrial pathway of apoptosis, which is particularly relevant in the context of diabetes-induced beta-cell dysfunction (Saxena etal., 2010).
4.3 Implications in retinitis pigmentosa
In their study, Xue et al. (2021) demonstrated that adeno-associated virus (AAV)-mediated overexpression of TXNIP in mouse models of retinitis pigmentosa (RP) prolonged cone survival and preserved vision by regulating cellular redox balance. TXNIP overexpression led to an adaptive increase in antioxidant responses, suggesting that controlled TXNIP activity can fine-tune oxidative stress to favor cellular resilience under pathological conditions. While excessive TXNIP activity has been associated with mitochondrial dysfunction and apoptosis, as observed in MI, this study highlights a nuanced role of TXNIP in mitigating stress-induced damage under certain conditions.
4.4 Implications in nephrotic syndrome
Mitochondrial TXNIP has been implicated in the pathogenesis of nephrotic syndrome, particularly in the context of oxidative stress and kidney injury. TXNIP is a key regulator of mitochondrial function, influencing redox balance and apoptosis by inhibiting thioredoxin, a critical antioxidant protein. In nephrotic syndrome, excessive TXNIP activity exacerbates mitochondrial dysfunction and oxidative stress, contributing to podocyte injury and proteinuria. A recent study demonstrated that CHOP-dependent shuttling of TXNIP to mitochondria amplifies oxidative damage and inflammation in kidney cells, thereby aggravating albuminuria and renal injury (Park et al., 2022). The authors found that blocking this pathway mitigates kidney injury by preserving mitochondrial integrity and reducing oxidative stress, highlighting the therapeutic potential of targeting mitochondrial TXNIP in nephrotic syndrome. These findings suggest that modulation of TXNIP could offer a novel strategy to protect kidney function in patients with nephrotic syndrome (Table 2).
Table 2. Pathophysiological implications of mitochondrial TXNIP in diseases.
5. Therapeutic potential of mitochondrial TXNIP/TBP-2
Mitochondrial TXNIP has emerged as a promising therapeutic target due to its central role in regulating oxidative stress, apoptosis, and metabolic disorders. By inhibiting mitochondrial thioredoxin-2 (TRX2), TXNIP disrupts redox homeostasis, leading to excessive reactive oxygen species (ROS) accumulation and subsequent activation of apoptotic and inflammatory pathways. This makes TXNIP a critical factor in the progression of diseases such as diabetes, myocardial infarction, neurodegeneration, and nephrotic syndrome. Targeting TXNIP holds potential for mitigating oxidative stress-induced damage, as evidenced by studies showing that TXNIP inhibition preserves mitochondrial integrity, reduces inflammation, and enhances cellular survival. Furthermore, its involvement in ferroptosis and mitophagy suggests that modulating TXNIP activity could be beneficial in conditions associated with mitochondrial dysfunction, such as diabetic retinopathy and cancer. By leveraging strategies that suppress TXNIP expression or disrupt its interaction with TRX2, novel therapeutic approaches could be developed to combat oxidative stress-related diseases and improve clinical outcomes.
6. Conclusions
The discovery of mitochondrial TXNIP has opened new avenues for understanding its multifaceted roles in cellular physiology and pathology. By regulating mitochondrial redox balance, TXNIP plays a crucial role in oxidative stress responses, apoptosis, cancer, and metabolism. The pathological consequences of reduced presence or loss of TXNIP should also be considered very carefully. As research progresses, targeting mitochondrial TXNIP could offer promising therapeutic strategies for oxidative stress-related diseases, including cancer and metabolic disorders. Further studies are warranted to fully elucidate the molecular mechanisms that lead the mitochondrial translocation of TXNIP and therapeutic potential.
Acknowledgements
The author would like to express his sincere gratitude to Professor Shinya Toyokuni, MD, PhD, Kyoto University (present location, Nagoya University), Japan, for supporting, guiding and supervising the first discovery (internationally) of TXNIP (TBP-2) inside the cellular mitochondria.
Source of funding
This work received no funding from internal or external sources.
Declaration by authors
The authors' guidelines were used to generate the manuscript with the assistance of ChatGPT, an artificial intelligence program developed by OpenAI (which included the information mining, drafting and even for verification). However, the authors are responsible for the content and accuracy of the manuscript.
Ethics approval statement
No ethical approval is required for this study.
Data availability
The raw data are available in corresponding author and ready to submit when ask for it.
Informed consent statement
No informed consent was required to conduct the study.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contribution
Khokon Kumar Dutta contributed to the design and writing of this review. The author critically reviewed the manuscript and agreed to submit final version of the article.



