Moringa oleifera, a member of the Moringaceae family and often referred to as the "miracle plant" or "tree of life," is a medicinal and nutrient-rich plant native to India, Bangladesh, Pakistan, and Afghanistan (Iqbal et al., 2006; Fahey et al., 2005). It is an evergreen tree that grows quickly even in poor soils, is well adapted to droughts, and may reach a height of up to 15 meters (Muhl et al., 2011). Almost every part of M. oleifera is utilized in South Asian traditional medicine to treat a variety of diseases, including diabetes, inflammation, hypertension, and viral infections (Verma et al., 2009). Owing to the abundance of phenolic acids and flavonoids, M. oleifera leaf extracts have been shown to demonstrate antioxidant activity both in vitro and in vivo (Atawodi et al., 2010). Fruits, roots, gums, flowers, and leaves are all often utilized to relieve inflammation (Manaheji et al., 2011). Phenols, proteins, magnesium, calcium, potassium, manganese, iron, copper, ascorbic acid, carotenoids, and tocopherols are all found in M. oleifera leaves. These substances have immunosuppressive properties and scavenge free radicals. In addition to having strong antioxidant and diuretic properties, seeds also contain antihyperlipidemic, anticancer, cardiovascular, liver dysfunction, and hematological disease properties (do Nascimento et al., 2017; Saini et al., 2016; Hekmat et al., 2015). According to pharmacological studies, M. oleifera has anti-microbial, hepatoprotective, immunomodulatory, antioxidant, hypoglycemic, hypotensive, and anticancer properties (Sudha et al., 2010; Mahajan et al., 2010; Anwar et al., 2007). These previously reported pharmacological properties bear evidence of the presence of numerous secondary plant metabolites such as vitamins, glycosides, sterols, flavonoids, phenolics, and alkaloids in M. oleifera (Auwal et al., 2013). The pharmacological potential of M. oleifera's leaves, seeds, and roots has been thoroughly studied in the past, but the plant's flowers have attracted relatively less attention. This is true even though M. oleifera flowers have long been utilized in traditional medicine due to their anti-inflammatory, antibacterial, and antioxidant qualities (Atawodi et al., 2010). In contrast to leaves and seeds, M. oleifera flowers are known to have a unique profile of bioactive substances, such as flavonoids, phenolic acids, and alkaloids. These substances are probably in charge of the flowers' distinct pharmacological properties, which have not yet been thoroughly investigated (Saini et al., 2016). According to preliminary research, M. oleifera flowers might show either equivalent or even better pharmacological action than other sections of the plant. For instance, compared to the leaves, the flowers have been claimed to have higher levels of certain antioxidants, such quercetin and kaempferol (do Nascimento et al., 2017). M. oleifera leaves and seeds have been extensively researched for their antidiarrheal, analgesic, and antioxidant properties. However, there is limited scientific evidence on the pharmacological activities of the flowers. The gap in the literature highlights the need of more investigation to confirm traditional use of M. oleifera flowers and investigate their possible source of new therapeutic compounds.
Medicinal plants serve as natural reservoirs of bioactive and therapeutic compounds, vital in disease prevention and enhancing human health. Traditional community medicine often uses them to treat or alleviate various ailments (Pawar et al., 2014; Rodrigues et al., 2011). Almost 80% of the total population of developing countries depends on complementary and traditional medicine for their basic medical needs according to the information from the World Health Organization (WHO) (Zhang et al., 2014). Due to benefits including less cost and broad acceptability from their long history of usage, the use of medicinal plants in treatments has continued to rise even now (Veiga Junior et al., 2005). Many phytochemicals, including alkaloids, glycosides, flavonoids, steroids, saponins, carbohydrates, gums, phenolic compounds, tannins, essential oils, volatile oils, etc. that are derived from plants have a wide range of pharmacological and therapeutic properties and are in charge of combating a variety of diseases (Islam et al., 2020; Islam et al., 2016; Akinmoladun et al., 2007).
Natural substances with antibacterial, analgesic, hypoglycemic, antioxidant, anti-cancer, and other qualities have drawn a lot of interest lately due to their potential health benefits. Research on various types of medicinal plants has consequently increased significantly to identify the active components responsible for these pharmacological benefits (Shahid et al., 2023). Diarrhea, pain, and oxidative stress-related disorders remain significant global health concerns, particularly in developing regions (Paul et al., 2024; Arunachalam et al., 2022). Synthetic drugs used to treat these conditions often come with side effects and limitations, driving the need for safer, plant-based alternatives (Chaachouay et al., 2024). In this context, medicinal plants like M. oleiferaoffer promising therapeutic potential. While previous studies have explored the medicinal properties of M. oleifera leaves and seeds, limited research has been conducted on its flowers, particularly in relation to their antidiarrheal, analgesic, and antioxidant activities (Rockwood et al., 2013, Kumar et al., 2010). Although the traditional applications of M. oleifera’s various parts have been scientifically verified, more research is required to investigate the pharmacological activity and isolate specific compounds responsible for these effects, which could lead to advanced studies and potential compound identification for future drug development. This study aims to explore the antidiarrheal, analgesic, and antioxidant potential of the methanolic extract of the M. oleifera flower via in vivo and in vitro methods. The findings of this study had important implications for both traditional medicine and modern pharmaceuticals. Results exhibited antidiarrheal, analgesic, and antioxidant properties of M. oleifera flower extract substantiate its traditional use in folk medicine, especially in areas with limited access to general healthcare. The aqueous extract of M. oleifera flowers exhibited significant antidiarrheal and central analgesic properties, whereas its ethyl acetate fraction revealed strong antioxidant activity, indicating potential for novel therapeutic agents. The study also emphasizes the potential of M. oleifera flowers as a cost-effective and accessible alternative to synthetic medications, which frequently result in adverse side effects. The findings could serve as a foundation for further research into bioactive compounds, potentially facilitating their use in pharmaceutical formulations.
2. Materials and methods
2.1 Ethical approval statement
This study was conducted following ethical guidelines, and approval was obtained from the Animal Ethics Committee of the Faculty of Biological Science, University of Dhaka (Approval No: 270/Biol. Sci.). All experimental procedures involving animals were carried out per the ARRIVE guidelines 2.0 and institutional ethical regulations to ensure the humane treatment of animals (Seltman, 2012).
2.2 Preparation of plant sample
This study was conducted at the Department of Pharmacy, Faculty of Biological Science, Islamic University, Kushtia, Bangladesh and carried out over 10 months, from February 2024 to November 2024. The fresh flower sample of M. oleifera was collected from Kushtia, Bangladesh and a botanist of Jahangirnagar University Herbarium verified the plant sample's authenticity with accession number (JUH- 10271). After being washed, freshly picked flowers were dried for a few days at room temperature and then baked at 40 °C. After drying, the sample was ground into powder and stored in an airtight container.
2.3 Extraction and fractionation
The dried powder of the sample was extracted in methanol using the cold extraction procedure. In a clean, beaker, 300 gm of the powder was transferred and soaked in about 900 ml of methanol for 2 weeks with periodical stirring. Then, the filtrate was separated and the solvent was completely removed using a rotary evaporator set to a low temperature (degrees) and pressure, condensing the filtrate into a dry crude extract. Then, the fractionation was carried out for 5.0 mg of extract, dissolved in 10% methanol, using n-hexane, chloroform, ethyl acetate, and aqueous depending upon the polarity and following the modified Kupchan partitioning process protocol (VanWagenen et al., 1993). The obtained fractions were n-hexane (NHF), Chloroform (CF), Ethyl acetate (EAF), and Aqueous fraction (AQF).
2.4 Drugs and reagents
All liquid reagents involved in the study were of analytical grade. Saline water (from Popular Pharmaceuticals Ltd.), loperamide, diclofenac sodium, and morphine (provided by Incepta Pharmaceuticals Ltd.) were also used.
2.5 Experimental animal
Swiss-albino mice (4-5 weeks old, both sexes) were used to evaluate the antidiarrheal, central analgesic, and peripheral analgesic effects of the flower extract. The experimental animals were maintained under controlled laboratory conditions to ensure consistency and well-being.
2.6 Preparation of oral dose
Two doses (200 mg/kg bw and 400 mg/kg bw) were administered to the experimental animals to assess the targeted pharmacological properties in the study (Rahman et al., 2023). A pilot investigation on M. oleifera flower extract indicated that lower doses were inadequate for notable pharmacological effects, resulting in the selection of larger doses of 200 mg/kg and 400 mg/kg for observable biological activity (Manaheji et al., 2011). All the experimental and standard doses for loperamide, diclofenac sodium, and morphine were prepared as a suspension form by adding suspending agents and normal saline (0.9% NaCl).
2.7 In vivo study design
2.7.1 Antidiarrheal activity test
This research focused on assessing the antidiarrheal properties of the methanolic extract of M. oleifera in a controlled experimental setting using a castor oil-induced diarrhea model in mice (Shoba et al., 2001). All mice were orally administered 1 mL of castor oil to induce diarrhea, followed by a 30-minute interval. The study included three groups for comparison: a negative control group, which received a 10 mL/kg body weight solution of Tween 80; a positive control group, which was treated with a 50 mg/kg body weight solution of Loperamide; and experimental groups that were administered the methanolic extract at two different dosages (200 and 400 mg/kg). The antidiarrheal activity was evaluated by recording the total fecal discharges produced by each mouse over a four-hour observation period. This approach allowed a direct comparison of the extract's efficacy with that of the negative and positive controls. The percent inhibition of defecation was calculated by the following formula,

2.7.2 Central analgesic activity test
The study aimed to evaluate the central analgesic activity of methanolic extracts of M. oleifera using the tail-flick test in mice (Martínez-González et al., 2017). This method involved administering the extracts at two dosages (200 mg/kg and 400 mg/kg) to determine their potential pain-relieving effects. The negative control group received an oral saline solution containing 1% Tween 80 and the positive control group was treated with morphine (2 mg/kg subcutaneously), a well-known analgesic. To induce pain, the distal 5 cm of the mice's tails was immersed in hot water, and the latency period for tail withdrawal was taken as a measure of the central analgesic activity. Observations were made at different time intervals: 0, 30, 60, and 90 minutes after administering the treatments.
2.7.3 Peripheral analgesic activity test
The peripheral analgesic effects of M. oleifera flower extract were tested using the acetic acid-induced writhing method (Tamrat et al., 2017). The pain was triggered by injecting 0.1 mL of acetic acid intraperitoneally and writhing, defined as the characteristic contraction response, was observed. The positive control group was treated orally with diclofenac sodium (5 mg/kg), while the test groups received oral doses of the plant extracts (200 and 400 mg/kg). Twisting movements were observed and recorded for five minutes after administering the treatments. The formula for calculating the percentage of inhibition of twisting was-

2.8 In vitro study: Antioxidant activity test
The antioxidant potential of the M. oleifera flower was investigated with the help of 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals (Brand-Williams et al., 1995). The samples, including extracts and control (Ascorbic acid), were prepared in concentrations ranging from 500 to 1 μg/mL. Two mL of each solution were mixed thoroughly with 3 mL DPPH solution (20 μg/mL) and preserved in a dark chamber for half an hour. To ensure accurate measurement of free radical scavenging activity, a blank control was prepared by mixing 2 mL of methanol with 3 mL of DPPH solution. This blank control was used to measure the baseline absorbance of the DPPH solution without any sample. Then, the absorbance of these solutions was observed using a UV spectrophotometer (at 517 nm). The DPPH free radical inhibition percentage values were calculated, and by plotting these values against respective concentrations, 50% inhibitory concentration values (IC50) were obtained. Lower IC50 values indicate stronger antioxidant activity. The percentage of inhibition of the free radical (I%) was calculated using the following formula:
I% = (1 – As /Ab) x 100
Where A b and A s are for absorbance of the blank and samples respectively.
2.9 Statistical analysis
The observational data, obtained from in vivo investigations, were presented as mean values with their associated standard error. To determine whether the statistically significant differences between test samples and negative control groups are present, the p-value for each tested group was calculated by un-pair t-test utilizing a student t-test calculator. The differences between the control and test groups were considered statistically significant if the p-value became less than 0.05.
3. Results
3.1 Antidiarrheal activity
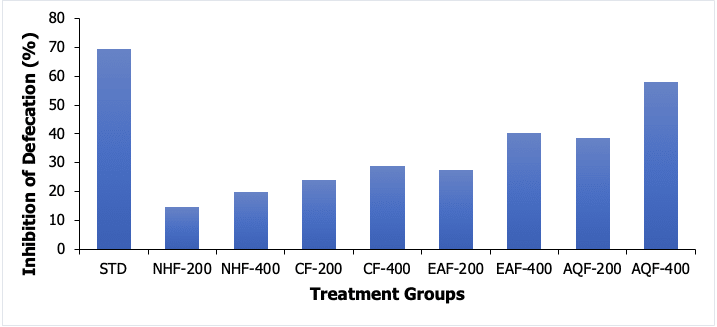
The outcomes of the antidiarrheal activity assessment revealed that the flower extract of M. oleifera possesses statistically significant activity (Table 1). The standard drug, loperamide (50 mg/kg), inhibited defecation by 69.31% (P<0.001). The n-hexane fraction (NHF) did not show significant results at either dose whereas other fractions showed significant activity. However, the highest activity was observed for an AQF at 400 mg dose with a 58.08% reduction in diarrheal frequency. A total of 40.27% and 38.72% reduction of defecation were measured for an ethyl acetated fraction at 400 mg/kg dose and an aqueous fraction at 200 mg/kg dose respectively shown in Figure 1.
Table 1. Antidiarrheal properties of different fractions of the flower extracts of M. oleifera.
3.2 Central analgesic activity
The different fractions of the methanolic extract of M. oleifera demonstrated significant central analgesia by extending the tail deflection time in albino mice, as shown in Table 2. At 30, 60, and 90 minutes after administration of medication samples in the albino mice, the percentage (%) elongation times were recorded. The study revealed that the n-hexane and aqueous fractions showed extremely significant central analgesic activity at both doses after 30, 60, and 90 minutes. The highest activity at 400 mg/kg of n-hexane and aqueous fractions, resulting in 136.38 ± 3.5 % and 181.71 ± 4.41 % as well as the best activity at 200 mg/kg of n-hexane and aqueous fractions with 120.35 ± 4.06 % and 100.16 ± 3.15 % tail immersion time respectively. On the contrary, the chloroform, ethyl acetate, and aqueous fractions do not show any central analgesic activity.
Table 2. Central analgesic activities of methanolic flower extract of M. oleifera. in mice by tail immersion method.
3.3. Peripheral analgesic activity
In the peripheral analgesic activity assay, diclofenac sodium was selected as a positive control (standard) in this assay. Compared to the control group, the standard drug inhibited 69.05% (P<0.001) of writhing (Figure 2). Chloroform-soluble and water-soluble fractions of the methanolic mother extract of M. oleifera flower exerted statistically significant outcomes at both doses though other fractions did not differ statistically compared to the control group indicated in Table 3. The most potent activity was produced by the chloroform fraction (CF) at both doses, with 40.48% (P<0.001) and 32.52% (P<0.001) writhing inhibition respectively. Aqueous soluble fractions exhibited a 23.81% (P<0.01) and 34.90% (P<0.001) reduction in the writhing count at lower and higher doses respectively shown in Figure 2.
Table 3. Peripheral analgesic activities of different fractions of the M. oleifera. flower in terms of the writhing count.

3.4 Antioxidant activity
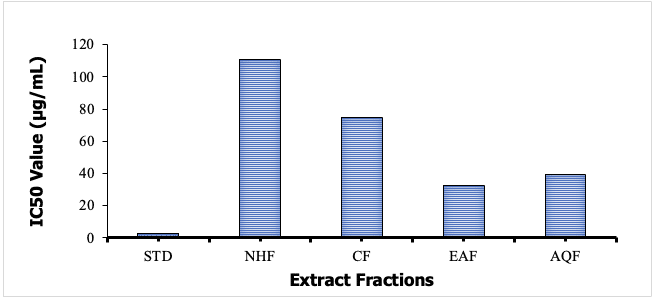
The ethyl acetate fraction (EAF) of M. oleifera exhibited the highest antioxidant activity among the test fractions with an IC50 value of 32.59±2.25 µg/mL. The AQF also showed antioxidant activity, achieving an IC50 value of 39.25± 3.38µg/mL in the DPPH free radical scavenging assay (Figure 3). The standard antioxidant, (ascorbic acid) exhibited significant potency with an IC50 value of 3.05±0.59 µg/mL. The IC50 values of the CF and the NHF were 74.55±2.83 µg/mL and 111.11±7.17 µg/mL, respectively.
4. Discussion
Plants with medicinal and food values are very blessing gifts of nature. Although this dual evidence drives human beings to take medicinal advantage of the plants along with dietary benefits, it can limit the uses of the plants as a food source if the medicinal-to-food value ratio becomes larger than one. The present study selected flower parts of M. oleifera, commonly used as a nutritious and safe plant with multiple evidence of pharmacological properties. This investigation also indicates that M. oleifera flower extract demonstrates significant antidiarrheal, analgesic, and antioxidant effects owing to its substantial phytochemical constituents, such as flavonoids, phenolic acids, alkaloids, and tannins (Gopalakrishnan et al., 2016; Akabari et al., 2011; Sreelatha et al., 2009; Verma et al., 2009)
The castor oil-induced diarrheal approach was performed to explore the antidiarrheal activity of the moringa flower. In the investigation, only secretory diarrhea was targeted to treat and no concern was placed on the pathogenic diarrhea caused by Salmonella, Clostridium, E. coli, Campylobacter, and Shigella (Dimka et al., 2022). So, this investigation determined only the intestinal motility inhibition activity of the phytochemicals present in the plant part. The outcomes indicate that the highest antidiarrheal activity was observed for the aqueous fraction of the flower extract at 400 mg/kg dose with 58.08% inhibition of defecation count. The second highest activity was exerted by ethyl acetate fraction with 40.27% inhibition of defecation at a larger dose. Overall results indicate that the aqueous, ethyl acetate and chloroform fractions exerted antidiarrheal potential. This result is in consistent with earlier research on M. oleifera leaves, which in animal models have also shown strong antidiarrheal properties. So, the findings showed that the flower extract's aqueous fraction considerably decreased diarrheal episodes, indicating that it contains bioactive substances that can alter intestinal motility. To assess the effectiveness of M. oleifera flower extract against diarrheal diseases caused on by bacterial, viral, or parasite infections, future studies should include models of pathogen-induced diarrhea.
The tail-flicking technique and acetic acid-based body writhing method were applied respectively to assess the central and peripheral analgesic activities of the flower extract of M. oleifera. The findings from the tail flicking technique indicate that the best activity was observed for aqueous fraction at 400 mg/kg dose with a 181.71 %-time elongation in the tail withdrawing. The second-highest activity was exerted by n-hexane fraction at 400 mg/kg dose with a 136.35 %-time elongation in the tail flicking. Overall results indicate that n-hexane-soluble and aqueous-soluble fractions showed statistically significant levels of central analgesic activity. However, the acetic acid-induced writhing test indicates that the maximum activity was induced from the chloroform soluble and aqueous fractions at 400 mg/kg dose with 40.48% and 34.9% writhing inhibition respectively. The entire outcomes of the induced writhing test suggest that both doses of the chloroform and aqueous fractions exhibited peripheral analgesic activity at a significant level. These findings align with prior research on M. oleifera leaves and roots, which have also documented substantial analgesic effects (Martínez-González et al., 2017). The results suggest that the flower extract contains bioactive compounds capable of modulating pain perception through both central and peripheral mechanisms.
The DPPH free radical scavenging activity was applied to measure the antioxidant potentialities of the flower extract of M. oleifera. The data was generated as a concentration required for 50% inhibition (IC50) of the applied DPPH to the sample. The best activities were observed for the ethyl acetate and aqueous fractions with the minimum IC50 values of 32.59 µg/mL and 39.25 µg/mL respectively. The minimum IC50 values indicate that these two fractions may contain some bioactive phytochemicals with free radicals inhibiting capacity. The previous study said that the leaves and seeds of M. oleiferahave significant antioxidant activity attributed to the presence of phenolic compounds and flavonoids that are correlated with our study (do Nascimento et al., 2017). So, these chemical constituents can be potent agents to prevent cancer generated from the harmful effects of free radicals (Didier et al., 2023). The elevated antioxidant activity of the floral extract indicates its potential as a significant source of natural antioxidants, suitable in the prevention and treatment of diseases associated with oxidative stress.
The present study had temperate methodological limitations. In the case of the antidiarrheal activity test, only secretory diarrhea was considered without targeting pathogenetic diarrhea. Again, the antioxidant activity was tested using DPPH free radicals only. Free radicals such as hydroxyl, nitric oxide free, and other natural free radicals were not considered for antioxidant assay (Tabassum et al., 2024).
5. Conclusion
Although other parts of M. oleifera such as leaves, barks and fruits have previous reports on many pharmacological properties, the present study unfold the pharmacological properties of the flower part. Findings from the in vivo and in vitro investigations drive us to conclude that the flower part of M. oleifera has potential antidiarrheal, analgesic, and antioxidant activities which align with its traditional uses in folk medicine. The aqueous fraction (AQF) of M. oleiferaflowers exhibited significant antidiarrheal and central analgesic effects, whilst the ethyl acetate fraction (EAF) had strong antioxidant activity, suggesting potential therapeutic uses. But the study was conducted with the crude extract only. This scientific study elucidates the pharmacological attributes of M. oleifera flower extract; but the exact mechanisms behind these effects remain unclear. The study assessed antidiarrheal activity by only castor oil-induced diarrhea, and yet it lacked a comprehensive phytochemical analysis. Future research should focus on identifying and characterizing bioactive compounds in M. oleifera flower extract, including mechanistic studies and alternative models, for its antidiarrheal, analgesic, and antioxidant properties. The isolation and identification of these bioactive phytochemicals can lead to develop the new drug candidate for the targeted diseases.
Acknowledgements
The authors express their gratefulness to the University of Dhaka, Bangladesh.
Source of funding
This research was conducted without any financial support from governmental, non-governmental, or private funding agencies. The study was entirely self-funded by the authors, and no specific grants were received for this research, authorship, or publication.
Data availability
The data underlying this article are available in the article. If detail data are required upon request to the corresponding author.
Informed consent statement
No informed consent was required to conduct the study.
Conflict of interest
The authors have no conflict in terms of financial or non-financial interests.
Authors’ contribution
Conceptualization, methodology, data curation, formal analysis, writing original draft: Md. Rasul Karim; investigation, data collection, validation: Md. Riaz Hosen; software, validation, editing, writing original draft: Md. Shamim; investigation, data analysis, methodology, draft writing: Md Alfaz Hossain; methodology, validation, visualization: Md. Saiful Islam; supervision, project administration, writing review, and editing: Md. Mizanur Rahman. All authors critically reviewed the manuscript and agreed to submit final version of the article.



